Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A chemist carefully measures the amount of heat needed to raise the temperature of a 1.21kg sample of a pure substance from 0.5C to 10.1C.

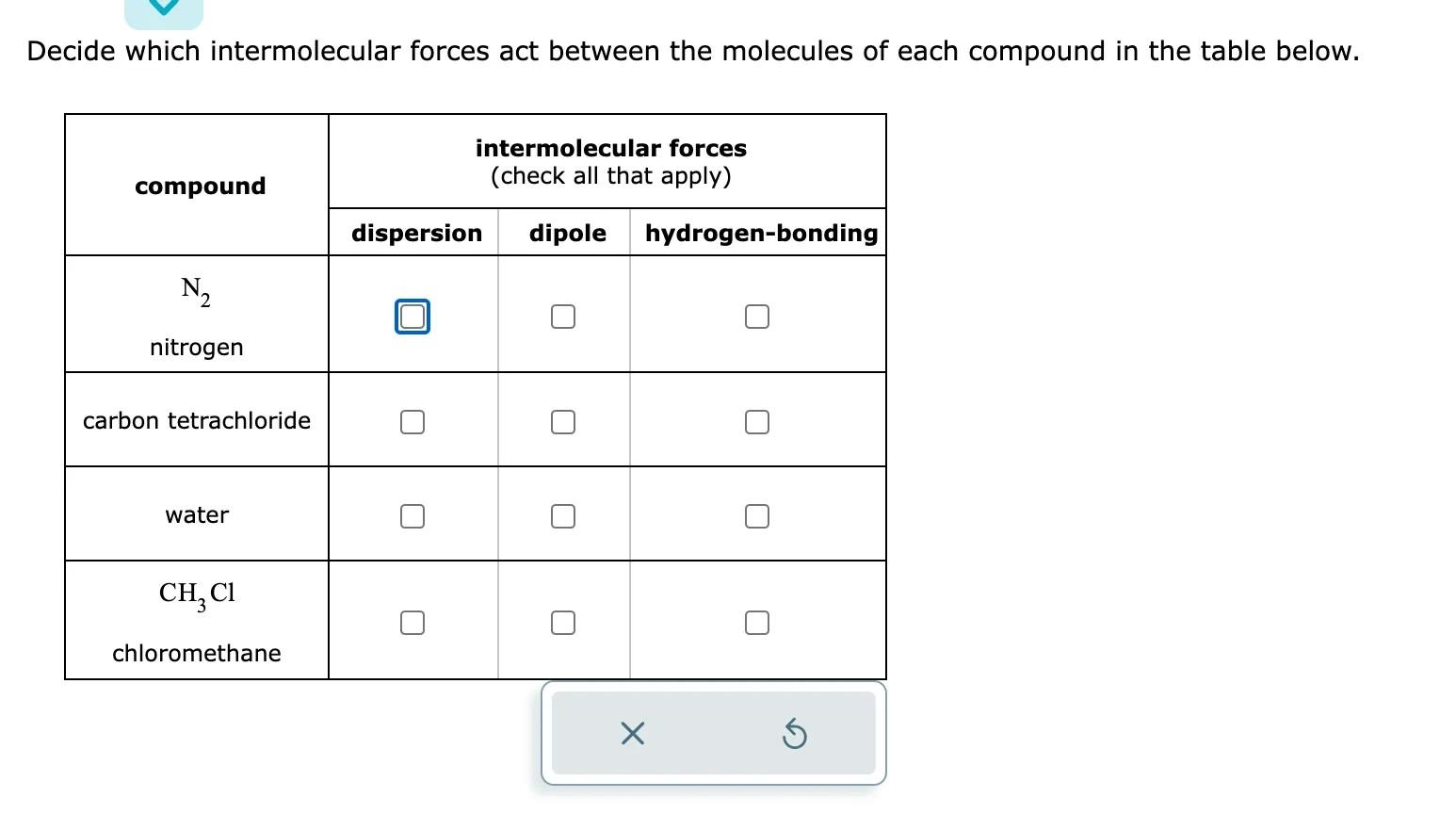
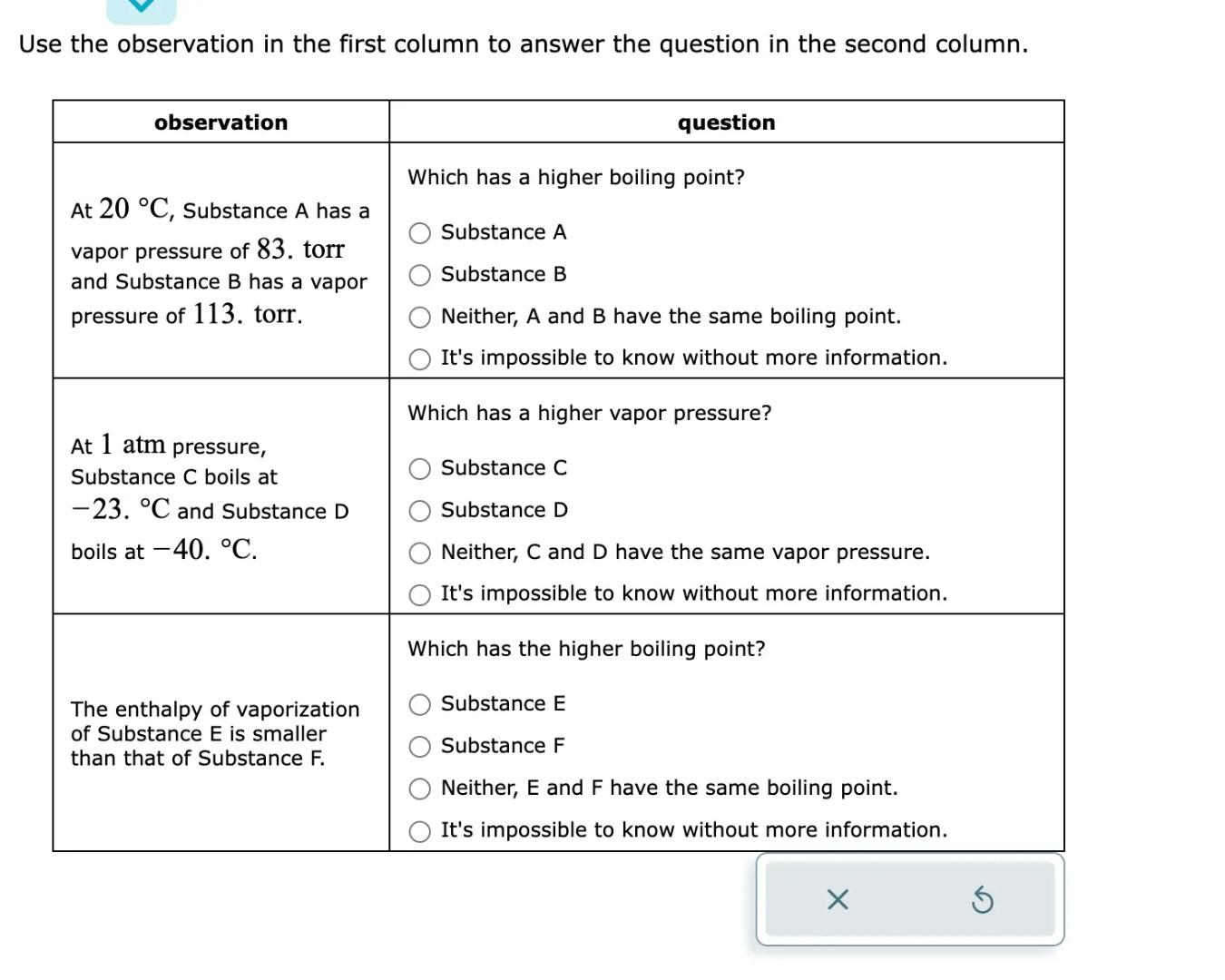
A chemist carefully measures the amount of heat needed to raise the temperature of a 1.21kg sample of a pure substance from 0.5C to 10.1C. The experiment shows that 10.kJ of heat are needed. What can the chemist report for the specific heat capacity of the substance? Round your answer to 2 significant digits. Decide which intermolecular forces act between the molecules of each compound in the table below. Use the observation in the first column to answer the question in the second column. \begin{tabular}{|l|l} \hline \multicolumn{1}{|c|}{ observation } & \\ \hline At 20C, Substance A has a & 1 \\ vapor pressure of 83 . torr \\ and Substance B has a vapor \\ pressure of 113 . torr. \end{tabular}
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


