Question
A chemist combines bromomethane and sodium methoxide in a polar aprotic solvent? What type of reaction is likely to occur? Hint: the figure below, which
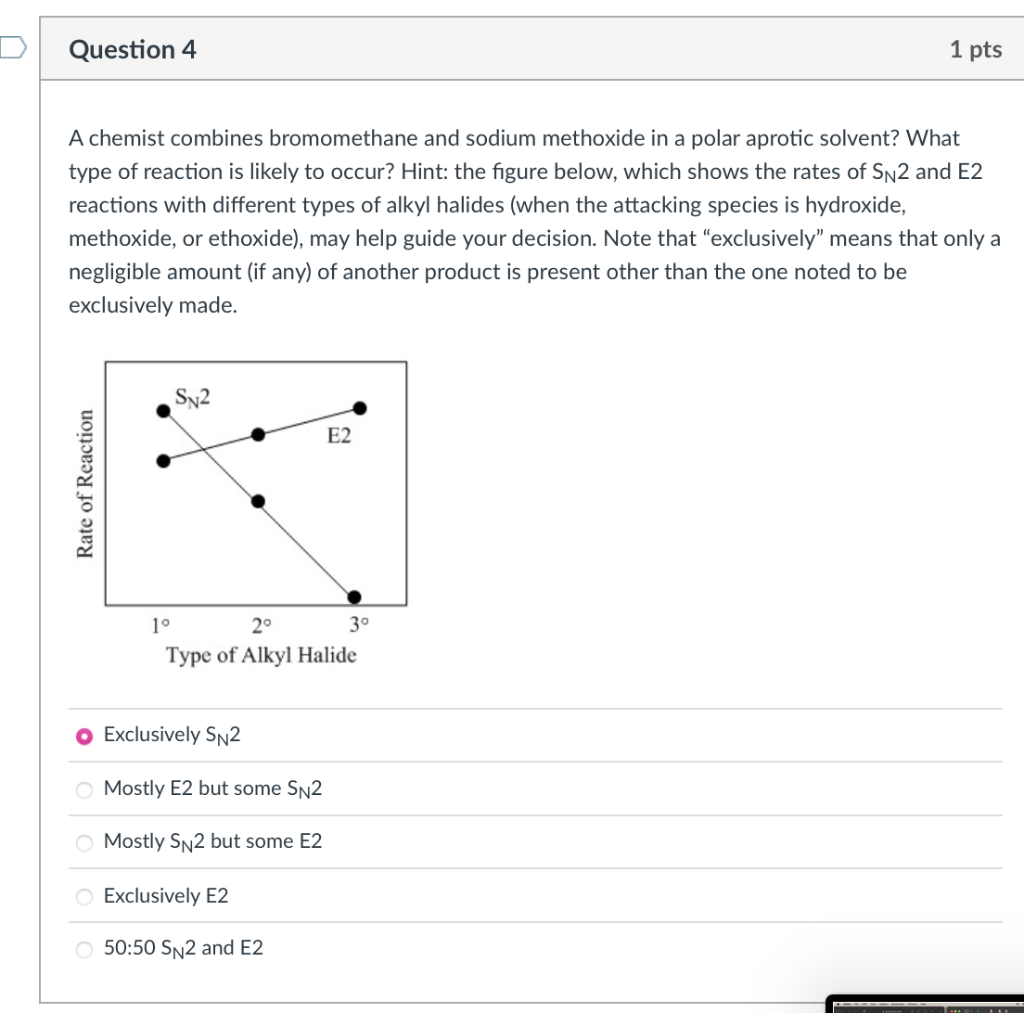
A chemist combines bromomethane and sodium methoxide in a polar aprotic solvent? What type of reaction is likely to occur? Hint: the figure below, which shows the rates of SN2 and E2 reactions with different types of alkyl halides (when the attacking species is hydroxide, methoxide, or ethoxide), may help guide your decision. Note that exclusively means that only a negligible amount (if any) of another product is present other than the one noted to be exclusively made.
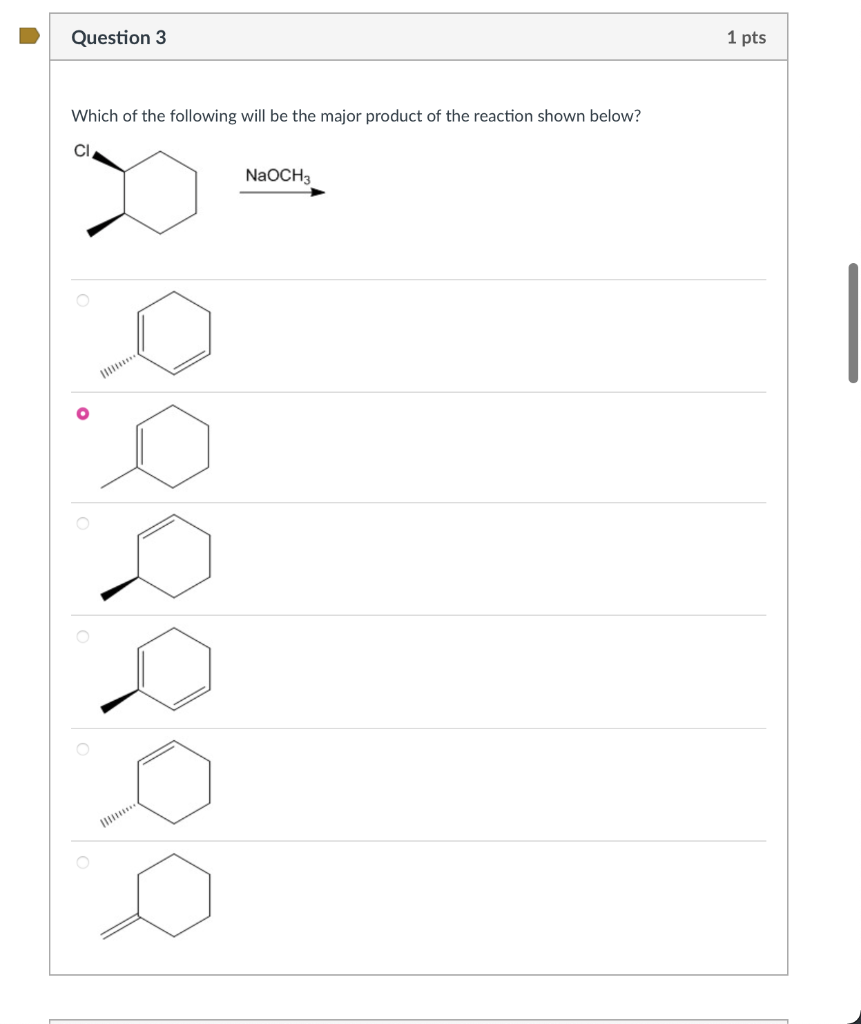
Which of the following will be the major product of the reaction shown below?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


