Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A chemist measures the energy change AH during the following reaction: CH(9)+20,(g) CO,(g)+2H,0(1) AH=-882. kJ Use the Information to answer the following questions. endothermic.
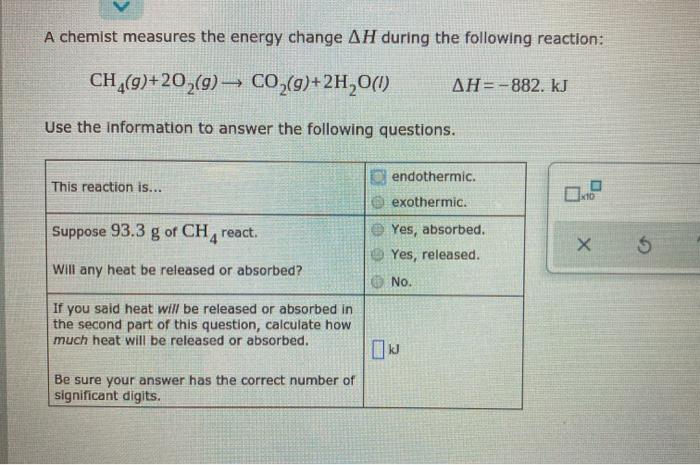
A chemist measures the energy change AH during the following reaction: CH(9)+20,(g) CO,(g)+2H,0(1) AH=-882. kJ Use the Information to answer the following questions. endothermic. This reaction is... 10 exothermic. Suppose 93.3 g of CH, react. Yes, absorbed. O Yes, released. WIll any heat be released or absorbed? O No. If you said heat will be released or absorbed in the second part of this question, calculate how much heat will be released or absorbed. Be sure your answer has the correct number of significant digits.
Step by Step Solution
★★★★★
3.39 Rating (165 Votes )
There are 3 Steps involved in it
Step: 1
2029 CO2 4 2H20e A1862 J a If AM 40 exotheomic rection If AHO endothermic veact...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


