Question
A chemist measures the enthalpy change AH during the following reaction: 2 CH3OH(1) + HSO4(1)(CH3)SO4(1) + 2 HO(1) AH=-15. kJ 2 Use this information
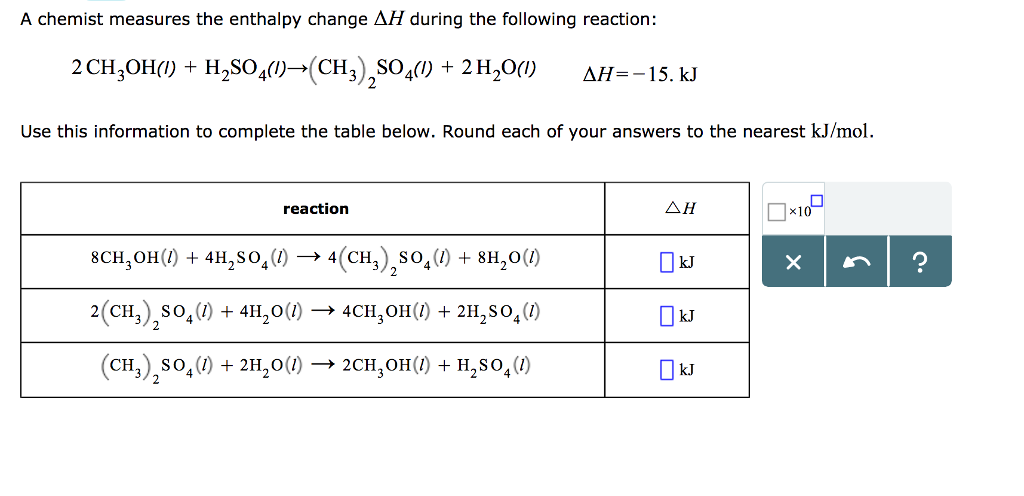
A chemist measures the enthalpy change AH during the following reaction: 2 CH3OH(1) + HSO4(1)(CH3)SO4(1) + 2 HO(1) AH=-15. kJ 2 Use this information to complete the table below. Round each of your answers to the nearest kJ/mol. reaction 8CHOH(/) + 4HSO4(1) 4(CH)SO4(1) + 8HO(1) 2(CH)SO4(1) + 4HO(1) 4CHOH(1) + 2HSO4 (1) (CH)SO4 (1) + 2HO(1) 2CHOH(1) + HSO4 (1) kJ kJ kJ x10 X ?
Step by Step Solution
3.32 Rating (146 Votes )
There are 3 Steps involved in it
Step: 1
D 2 CHOH 1 H 504 1 CH3 Soy 1 9H10 1 OH15KJ O reverse reaction 2 CH3 Soy ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Cambridge International AS And A Level Chemistry Coursebook
Authors: Lawrie Ryan, Roger Norris
2nd Edition
1316637735, 978-1316637739
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



