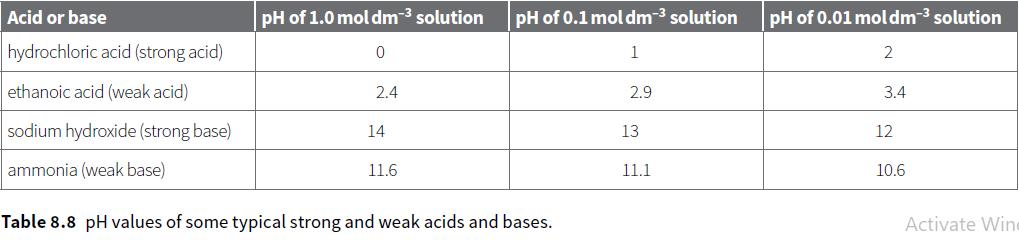
a. The pH of a solution depends on the hydrogen ion (hydroxonium ion) concentration. Which concentration of
Question:
a. The pH of a solution depends on the hydrogen ion (hydroxonium ion) concentration. Which concentration of ethanoic acid in Table 8.8 has the highest concentration of hydrogen ions in solution?
b. Which acid or alkali in Table 8.8 has the highest concentration of hydroxide ions?
c. Explain why a solution of 0.1moldm–3 ethanoic acid has a lower electrical conductivity than a solution of 0.1moldm–3 hydrochloric acid.
d. Both hydrochloric acid and ethanoic acid react with magnesium. The rate of reaction of 1.0moldm–3 hydrochloric acid with magnesium is much faster than the rate of reaction of 1.0moldm–3 ethanoic acid. Explain why.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris
Question Posted:





