Answered step by step
Verified Expert Solution
Question
1 Approved Answer
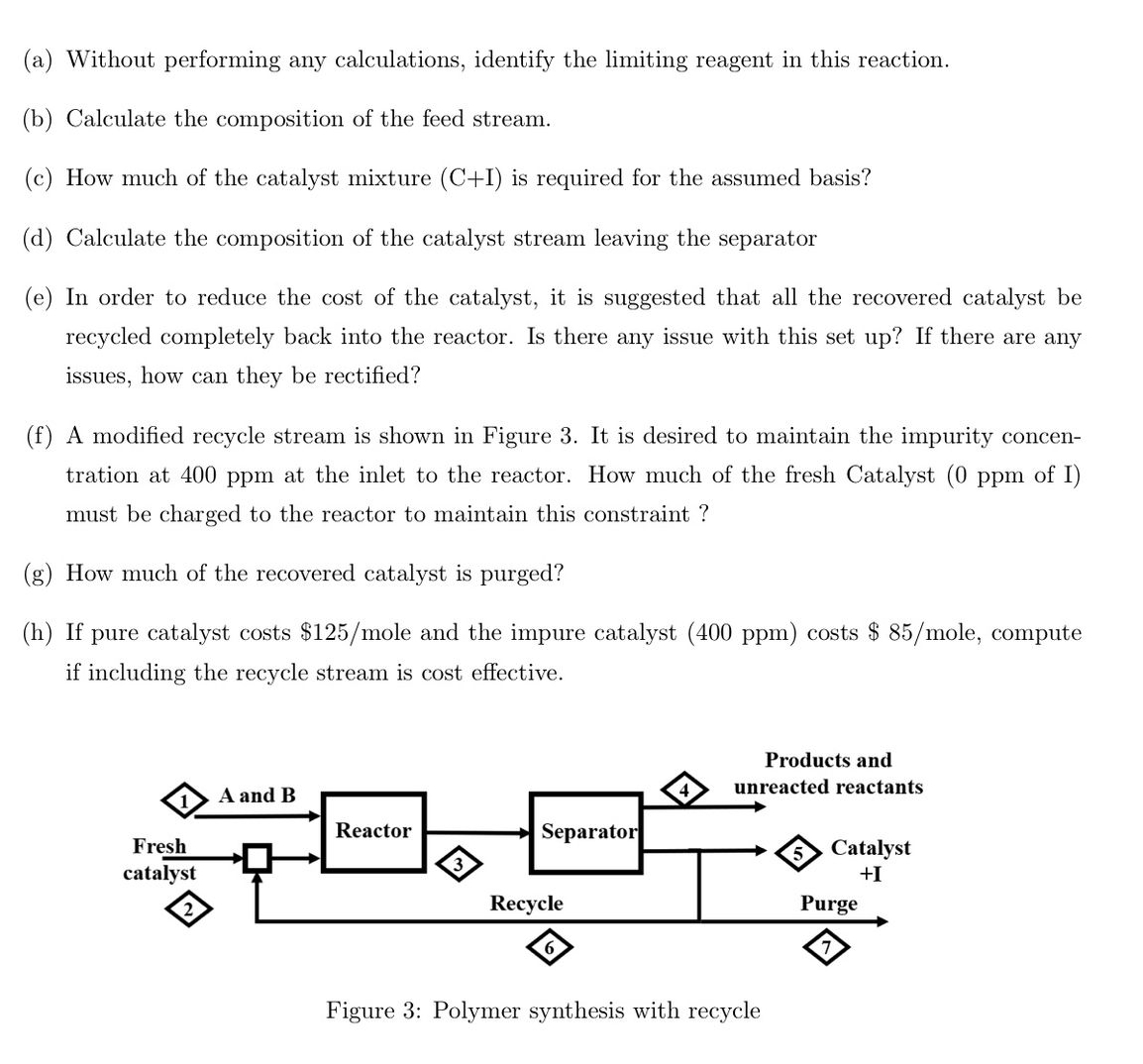
A co - polymer A B 2 is to be made from its constituent monomers A and B according to the equation A + 2
A copolymer is to be made from its constituent monomers A and according to the equation
where is a catalyst required for the reaction. It is known that the reaction also generates an impurity I at a rate of moles of mole of generated. A manufacturing process is set up in such a way that a feed stream containing pure A and B are sent to the reactor along with another stream of catalyst C which contains ppm of I. It is known that a excess of B has been charged into the reactor. The exit stream from the reactor is sent to a separator which generates a product
stream containing and any unreacted reactants and a catalyst stream containing the recovered catalyst and any impurities.
Assuming a basis of molesh of product and steady state,
a Without performing any calculations, identify the limiting reagent in this reaction.
b Calculate the composition of the feed stream.
c How much of the catalyst mixture is required for the assumed basis?
d Calculate the composition of the catalyst stream leaving the separator
e In order to reduce the cost of the catalyst, it is suggested that all the recovered catalyst be recycled completely back into the reactor. Is there any issue with this set up If there are any issues, how can they be rectified?
f A modified recycle stream is shown in Figure It is desired to maintain the impurity concentration at at the inlet to the reactor. How much of the fresh Catalyst of I must be charged to the reactor to maintain this constraint?
g How much of the recovered catalyst is purged?
h If pure catalyst costs $ mole and the impure catalyst ppm costs $ mole, compute if including the recycle stream is cost effective.
Figure : rolymer syntnesis witn recycie
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


