Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A combustion test was performed on a mixture of H and O2 in a steady flow reactor at a pressure of 20 atm. The
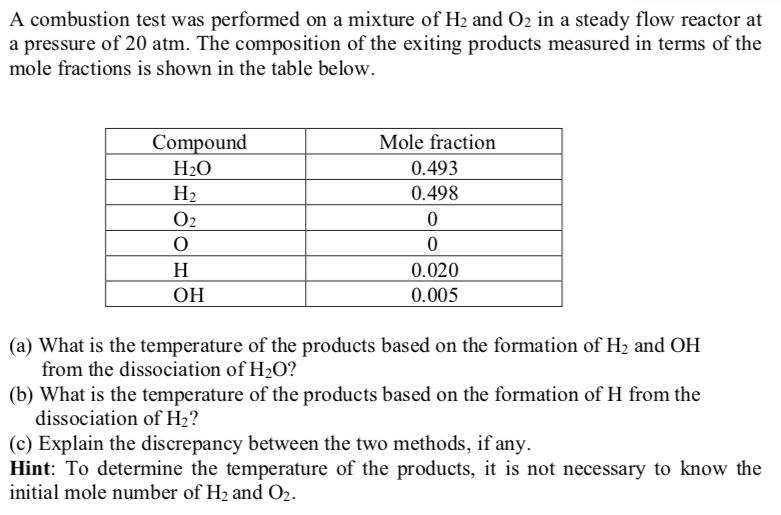
A combustion test was performed on a mixture of H and O2 in a steady flow reactor at a pressure of 20 atm. The composition of the exiting products measured in terms of the mole fractions is shown in the table below. Compound HO H 0 O H OH Mole fraction 0.493 0.498 0 0 0.020 0.005 (a) What is the temperature of the products based on the formation of H and OH from the dissociation of HO? (b) What is the temperature of the products based on the formation of H from the dissociation of H? (c) Explain the discrepancy between the two methods, if any. Hint: To determine the temperature of the products, it is not necessary to know the initial mole number of H and O2.
Step by Step Solution
There are 3 Steps involved in it
Step: 1
a nH2O 0493 0493 0498 0020 0005 0493 1016 0484 nH2 0498 0493 0498 0020 0005 0498 1016 0488 nOH 0020 0493 0498 0020 0005 0020 1016 0019 nO 0005 0493 0498 0020 0005 0005 1016 0005 DG0f H2O 2418 kJmol DG...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


