Answered step by step
Verified Expert Solution
Question
1 Approved Answer
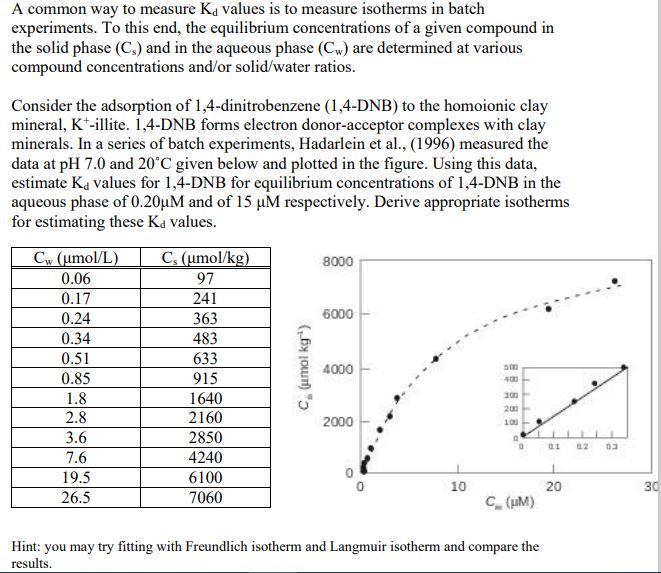
A common way to measure K d values is to measure isotherms in batch experiments. To this end, the equilibrium concentrations of a given compound
A common way to measure values is to measure isotherms in batch
experiments. To this end, the equilibrium concentrations of a given compound in
the solid phase and in the aqueous phase are determined at various
compound concentrations andor solidwater ratios.
Consider the adsorption of dinitrobenzene DNB to the homoionic clay
mineral, illite. DNB forms electron donoracceptor complexes with clay
minerals. In a series of batch experiments, Hadarlein et al measured the
data at and given below and plotted in the figure. Using this data,
estimate values for DNB for equilibrium concentrations of DNB in the
aqueous phase of and of respectively. Derive appropriate isotherms
for estimating these values.
Hint: you may try fitting with Freundlich isotherm and Langmuir isotherm and compare the
results.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


