Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A compound is 80.0% carbon and 20.0% hydrogen by mass. Assume you have a 100.-g sample of this compound. Part A How many grams
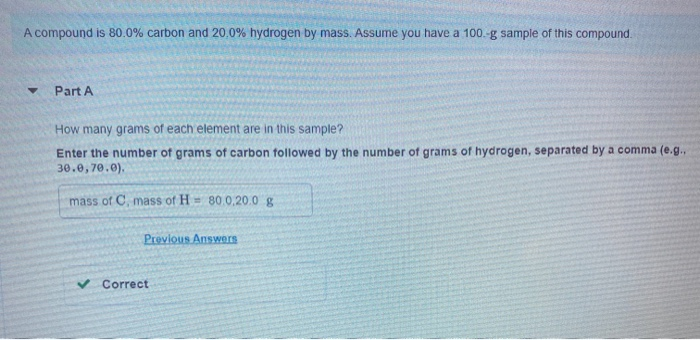

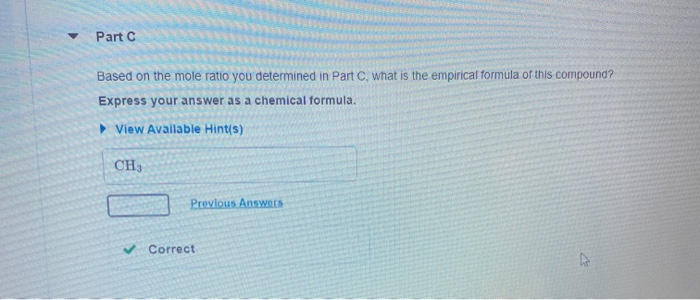
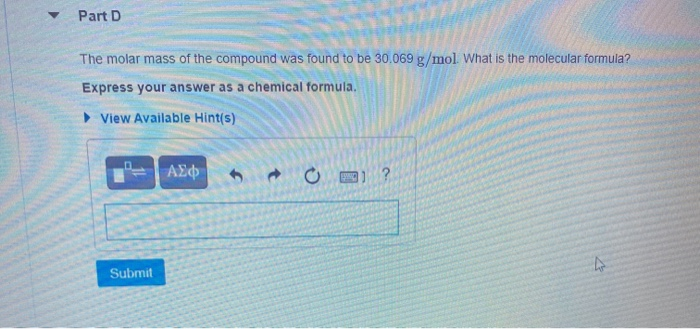
A compound is 80.0% carbon and 20.0% hydrogen by mass. Assume you have a 100.-g sample of this compound. Part A How many grams of each element are in this sample? Enter the number of grams of carbon followed by the number of grams of hydrogen, separated by a comma (e.g.. 30.0,70.0). mass of C, mass of H 80.0.20.0 g Previous Answers Correct Part B How many moles of each element are in this sample? Enter the number of moles of carbon followed by the number of moles of hydrogen, separated by a comma. View Available Hint(s) moles of C, moles of H= 6.66,19.8 mol Previous Answers Correct Part C Based on the mole ratio you determined in Part C, what is the empirical formula of this compound? Express your answer as a chemical formula. View Available Hint(s) CH3 Previous Answers Correct N Part D The molar mass of the compound was found to be 30.069 g/mol. What is the molecular formula? Express your answer as a chemical formula. View Available Hint(s) Submit
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


