Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A compressed-air storage system uses excess electricity to power a compressor that fills a tank with pressurized air. The tank has a volume of
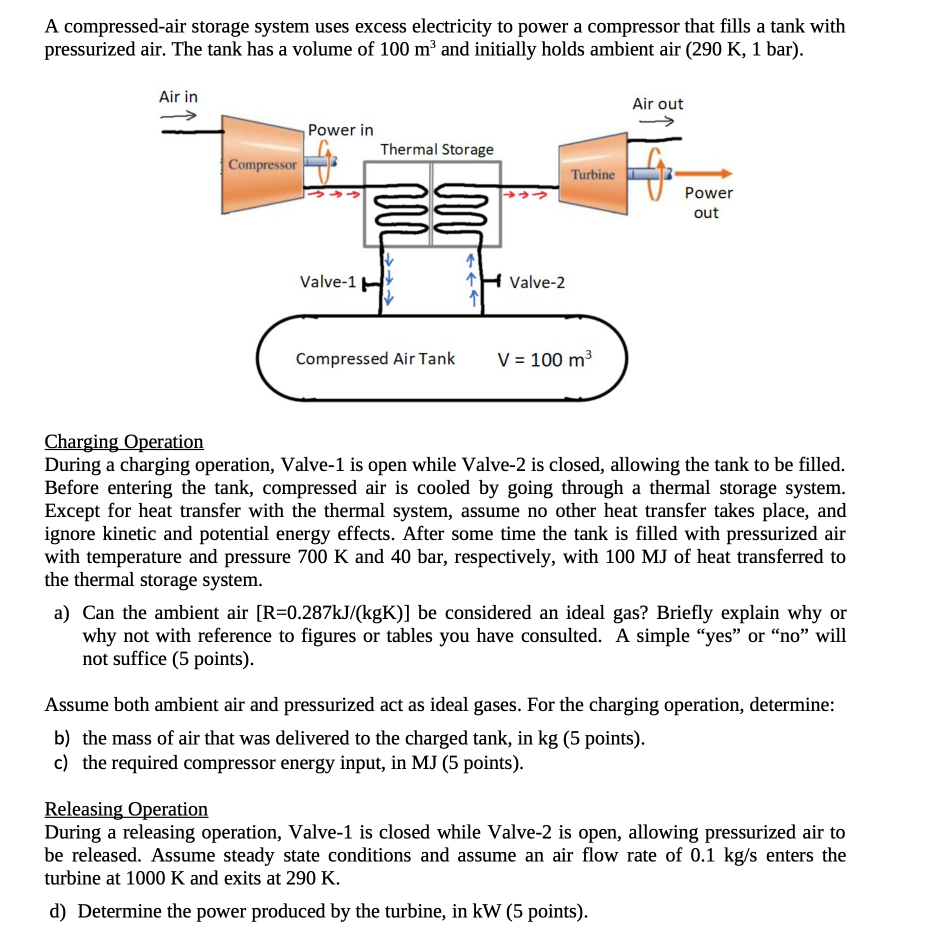
A compressed-air storage system uses excess electricity to power a compressor that fills a tank with pressurized air. The tank has a volume of 100 m and initially holds ambient air (290 K, 1 bar). Air in Compressor Power in Thermal Storage Valve-1 H Compressed Air Tank Valve-2 Turbine V = 100 m Air out Power out Charging Operation During a charging operation, Valve-1 is open while Valve-2 is closed, allowing the tank to be filled. Before entering the tank, compressed air is cooled by going through a thermal storage system. Except for heat transfer with the thermal system, assume no other heat transfer takes place, and ignore kinetic and potential energy effects. After some time the tank is filled with pressurized air with temperature and pressure 700 K and 40 bar, respectively, with 100 MJ of heat transferred to the thermal storage system. a) Can the ambient air [R=0.287kJ/(kgK)] be considered an ideal gas? Briefly explain why or why not with reference to figures or tables you have consulted. A simple "yes" or "no" will not suffice (5 points). Assume both ambient air and pressurized act as ideal gases. For the charging operation, determine: b) the mass of air that was delivered to the charged tank, in kg (5 points). c) the required compressor energy input, in MJ (5 points). Releasing Operation During a releasing operation, Valve-1 is closed while Valve-2 is open, allowing pressurized air to be released. Assume steady state conditions and assume an air flow rate of 0.1 kg/s enters the turbine at 1000 K and exits at 290 K. d) Determine the power produced by the turbine, in kW (5 points).
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Solutions 1 air can be treated as an id...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


