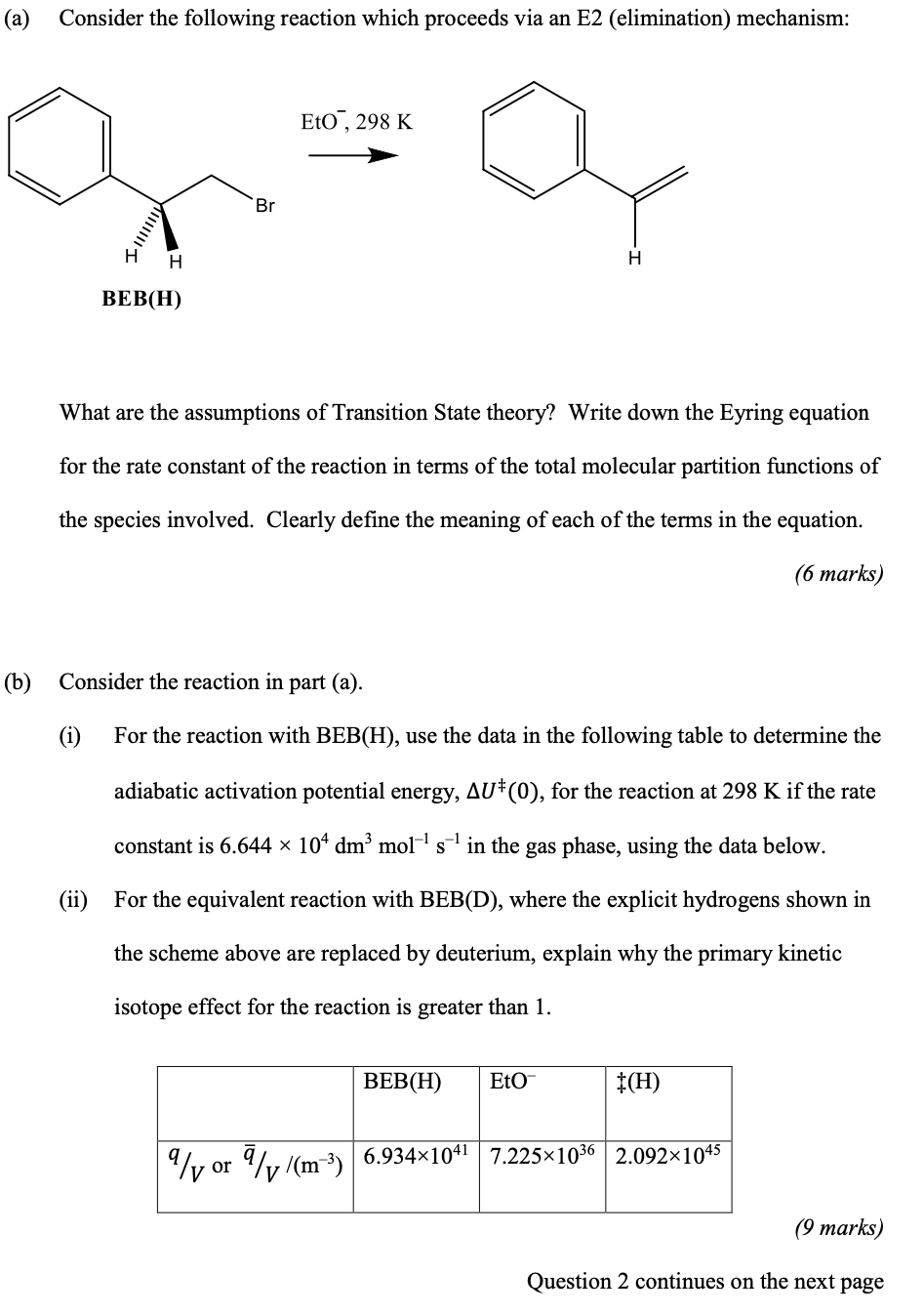

(a) Consider the following reaction which proceeds via an E2 (elimination) mechanism: Eto, 298 K Br H H BEB(H) What are the assumptions of Transition State theory? Write down the Eyring equation for the rate constant of the reaction in terms of the total molecular partition functions of the species involved. Clearly define the meaning of each of the terms in the equation. (6 marks) (b) Consider the reaction in part (a). (i) For the reaction with BEB(H), use the data in the following table to determine the adiabatic activation potential energy, Aut(o), for the reaction at 298 K if the rate constant is 6.644 x 104 dm' mol's ' in the gas phase, using the data below. (ii) For the equivalent reaction with BEB(D), where the explicit hydrogens shown in the scheme above are replaced by deuterium, explain why the primary kinetic isotope effect for the reaction is greater than 1. BEB(H) Eto #(H) Yor W/(m) 6.934x1041 7.225*1036 2.092x1045 (9 marks) Question 2 continues on the next page Question 2 continued (c) Consider the following two reactions: Cl + H-Br + Cl-H + Br (1) Br + H-I Br-H+I (2) (i) For the first reaction, the adiabatic activation energy and the adiabatic reaction energies are 3.3 and -65.4 kJ mol-' respectively. Use appropriate sketches to describe a trajectory with the highest probability of reaction and explain whether the reaction is expected to have an early or late transition state. (ii) For the second reaction the corresponding energies are 2.9 and 67.7 kJ mol-1. Using the harmonic vibrational energies in the following table, and neglecting any consideration of rotational energy, explain why the observed ratios of the energy of the products in the v=2 vibrational state relative to the products in the v=l state, P(v=2)/P(v=1), are approximately 0.3 and 1.2 for the two reactions respectively. H-C1 H-Br H-I Nhc /(kJ mol-1) 35.8 31.7 27.6 (5 Marks) 3. Answer ALL parts. (a) (i) Distinguish between spontaneous and stimulated emission. (3 marks) (ii) Explain why photoisomerisation between the cis- and trans-form of a molecule containing a single C=C bond does not necessarily lead to an equal mixture of the two isomers. (2 marks) (b) Draw a Jablonski diagram showing the electronic states So, S1, S2, T1 and their vibrational sub-levels. Indicate on your diagram the main photophysical processes that can occur from these various states. Quenching can be neglected. (6 marks) (ii) For a specific molecule X, the photophysical process with the largest quantum yield is found to be luminescence with a lifetime of around 10-9 seconds. What would the relative magnitudes of the rate constants for the processes shown in part (i) need to be for this to be the case? (4 marks) Compare and contrast the requirements for light sources used for gas-phase high- resolution spectroscopy with those for gas-phase reaction dynamics measurements. (5 marks) (a) Consider the following reaction which proceeds via an E2 (elimination) mechanism: Eto, 298 K Br H H BEB(H) What are the assumptions of Transition State theory? Write down the Eyring equation for the rate constant of the reaction in terms of the total molecular partition functions of the species involved. Clearly define the meaning of each of the terms in the equation. (6 marks) (b) Consider the reaction in part (a). (i) For the reaction with BEB(H), use the data in the following table to determine the adiabatic activation potential energy, Aut(o), for the reaction at 298 K if the rate constant is 6.644 x 104 dm' mol's ' in the gas phase, using the data below. (ii) For the equivalent reaction with BEB(D), where the explicit hydrogens shown in the scheme above are replaced by deuterium, explain why the primary kinetic isotope effect for the reaction is greater than 1. BEB(H) Eto #(H) Yor W/(m) 6.934x1041 7.225*1036 2.092x1045 (9 marks) Question 2 continues on the next page Question 2 continued (c) Consider the following two reactions: Cl + H-Br + Cl-H + Br (1) Br + H-I Br-H+I (2) (i) For the first reaction, the adiabatic activation energy and the adiabatic reaction energies are 3.3 and -65.4 kJ mol-' respectively. Use appropriate sketches to describe a trajectory with the highest probability of reaction and explain whether the reaction is expected to have an early or late transition state. (ii) For the second reaction the corresponding energies are 2.9 and 67.7 kJ mol-1. Using the harmonic vibrational energies in the following table, and neglecting any consideration of rotational energy, explain why the observed ratios of the energy of the products in the v=2 vibrational state relative to the products in the v=l state, P(v=2)/P(v=1), are approximately 0.3 and 1.2 for the two reactions respectively. H-C1 H-Br H-I Nhc /(kJ mol-1) 35.8 31.7 27.6 (5 Marks) 3. Answer ALL parts. (a) (i) Distinguish between spontaneous and stimulated emission. (3 marks) (ii) Explain why photoisomerisation between the cis- and trans-form of a molecule containing a single C=C bond does not necessarily lead to an equal mixture of the two isomers. (2 marks) (b) Draw a Jablonski diagram showing the electronic states So, S1, S2, T1 and their vibrational sub-levels. Indicate on your diagram the main photophysical processes that can occur from these various states. Quenching can be neglected. (6 marks) (ii) For a specific molecule X, the photophysical process with the largest quantum yield is found to be luminescence with a lifetime of around 10-9 seconds. What would the relative magnitudes of the rate constants for the processes shown in part (i) need to be for this to be the case? (4 marks) Compare and contrast the requirements for light sources used for gas-phase high- resolution spectroscopy with those for gas-phase reaction dynamics measurements









