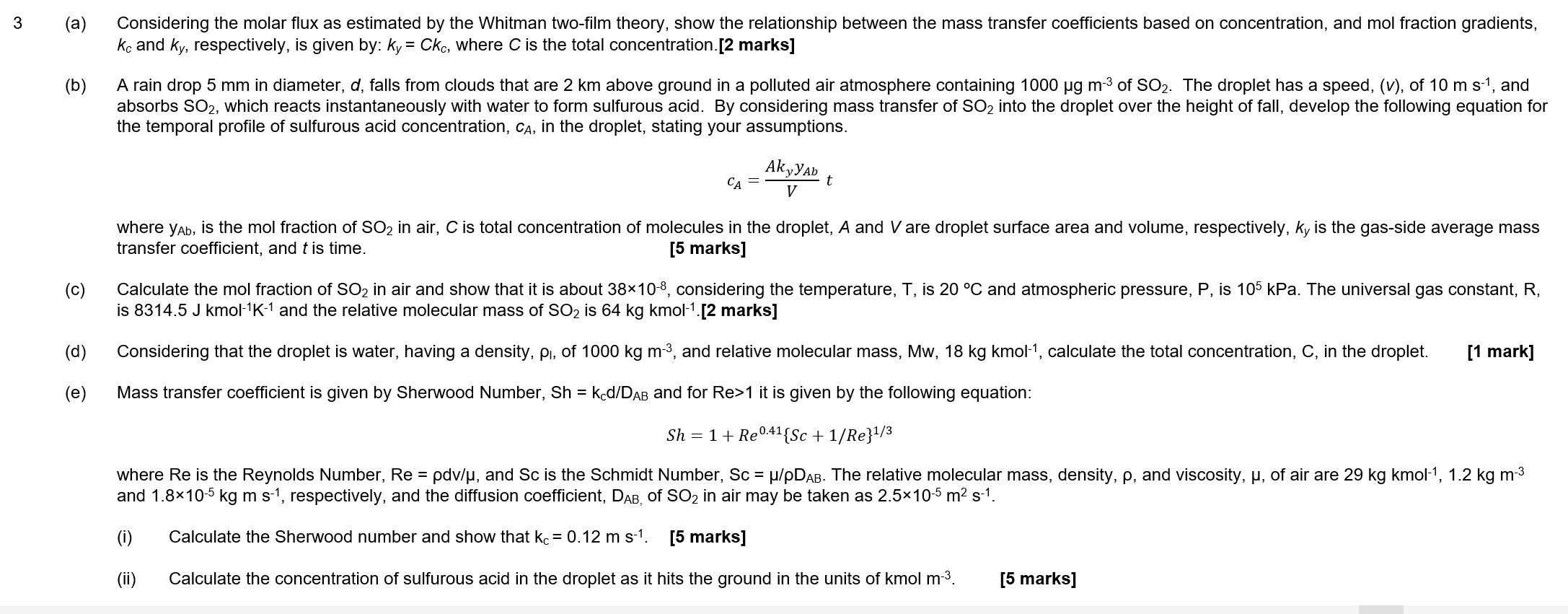
(a) Considering the molar flux as estimated by the Whitman two-film theory, show the relationship between the mass transfer coefficients based on concentration, and mol fraction gradients, kc and ky, respectively, is given by: ky=Ckc, where C is the total concentration.[2 marks] (b) A rain drop 5mm in diameter, d, falls from clouds that are 2km above ground in a polluted air atmosphere containing 1000mm3 of SO2. The droplet has a speed, ( v ), of 10mss1, and absorbs SO2, which reacts instantaneously with water to form sulfurous acid. By considering mass transfer of SO2 into the droplet over the height of fall, develop the following equation for the temporal profile of sulfurous acid concentration, CA, in the droplet, stating your assumptions. cA=VAkyyAbt where yAb, is the mol fraction of SO2 in air, C is total concentration of molecules in the droplet, A and V are droplet surface area and volume, respectively, ky is the gas-side average mass transfer coefficient, and t is time. [5 marks] (c) Calculate the mol fraction of SO2 in air and show that it is about 38108, considering the temperature, T, is 20C and atmospheric pressure, P, is 105kPa. The universal gas constant, R, is 8314.5Jkmol1K1 and the relative molecular mass of SO2 is 64kgkmol1 [2 marks] (d) Considering that the droplet is water, having a density, I, of 1000kgm3, and relative molecular mass, Mw,18kgkmol1, calculate the total concentration, C, in the droplet. [1 mark] (e) Mass transfer coefficient is given by Sherwood Number, Sh=kCd/DAB and for Re>1 it is given by the following equation: Sh=1+Re0.41{Sc+1/Re}1/3 where Re is the Reynolds Number, Re=dv/, and Sc is the Schmidt Number, Sc=/DAB. The relative molecular mass, density, , and viscosity, , of air are 29kgkmol1,1.2kgm3 and 1.8105kgms1, respectively, and the diffusion coefficient, DAB, of SO2 in air may be taken as 2.5105m2s1. (i) Calculate the Sherwood number and show that kc=0.12ms1. [5 marks] (ii) Calculate the concentration of sulfurous acid in the droplet as it hits the ground in the units of kmolm3. [5 marks] (a) Considering the molar flux as estimated by the Whitman two-film theory, show the relationship between the mass transfer coefficients based on concentration, and mol fraction gradients, kc and ky, respectively, is given by: ky=Ckc, where C is the total concentration.[2 marks] (b) A rain drop 5mm in diameter, d, falls from clouds that are 2km above ground in a polluted air atmosphere containing 1000mm3 of SO2. The droplet has a speed, ( v ), of 10mss1, and absorbs SO2, which reacts instantaneously with water to form sulfurous acid. By considering mass transfer of SO2 into the droplet over the height of fall, develop the following equation for the temporal profile of sulfurous acid concentration, CA, in the droplet, stating your assumptions. cA=VAkyyAbt where yAb, is the mol fraction of SO2 in air, C is total concentration of molecules in the droplet, A and V are droplet surface area and volume, respectively, ky is the gas-side average mass transfer coefficient, and t is time. [5 marks] (c) Calculate the mol fraction of SO2 in air and show that it is about 38108, considering the temperature, T, is 20C and atmospheric pressure, P, is 105kPa. The universal gas constant, R, is 8314.5Jkmol1K1 and the relative molecular mass of SO2 is 64kgkmol1 [2 marks] (d) Considering that the droplet is water, having a density, I, of 1000kgm3, and relative molecular mass, Mw,18kgkmol1, calculate the total concentration, C, in the droplet. [1 mark] (e) Mass transfer coefficient is given by Sherwood Number, Sh=kCd/DAB and for Re>1 it is given by the following equation: Sh=1+Re0.41{Sc+1/Re}1/3 where Re is the Reynolds Number, Re=dv/, and Sc is the Schmidt Number, Sc=/DAB. The relative molecular mass, density, , and viscosity, , of air are 29kgkmol1,1.2kgm3 and 1.8105kgms1, respectively, and the diffusion coefficient, DAB, of SO2 in air may be taken as 2.5105m2s1. (i) Calculate the Sherwood number and show that kc=0.12ms1. [5 marks] (ii) Calculate the concentration of sulfurous acid in the droplet as it hits the ground in the units of kmolm3. [5 marks]







