Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A constant volume gas thermometer of volume 1x10-3 m3 contains 0.05 mol of gas. It is assumed that the gas obeys the perfect gas
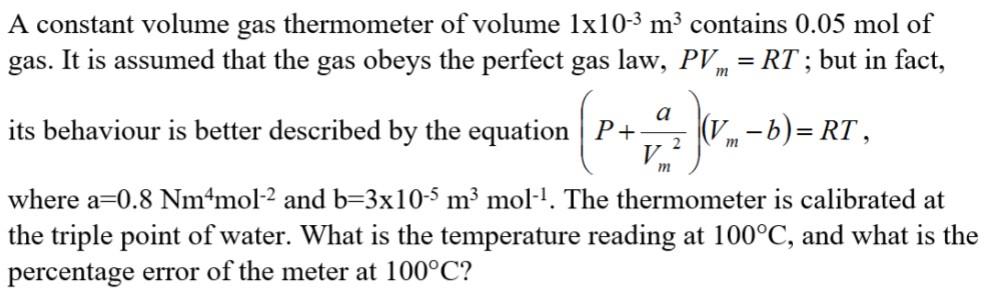
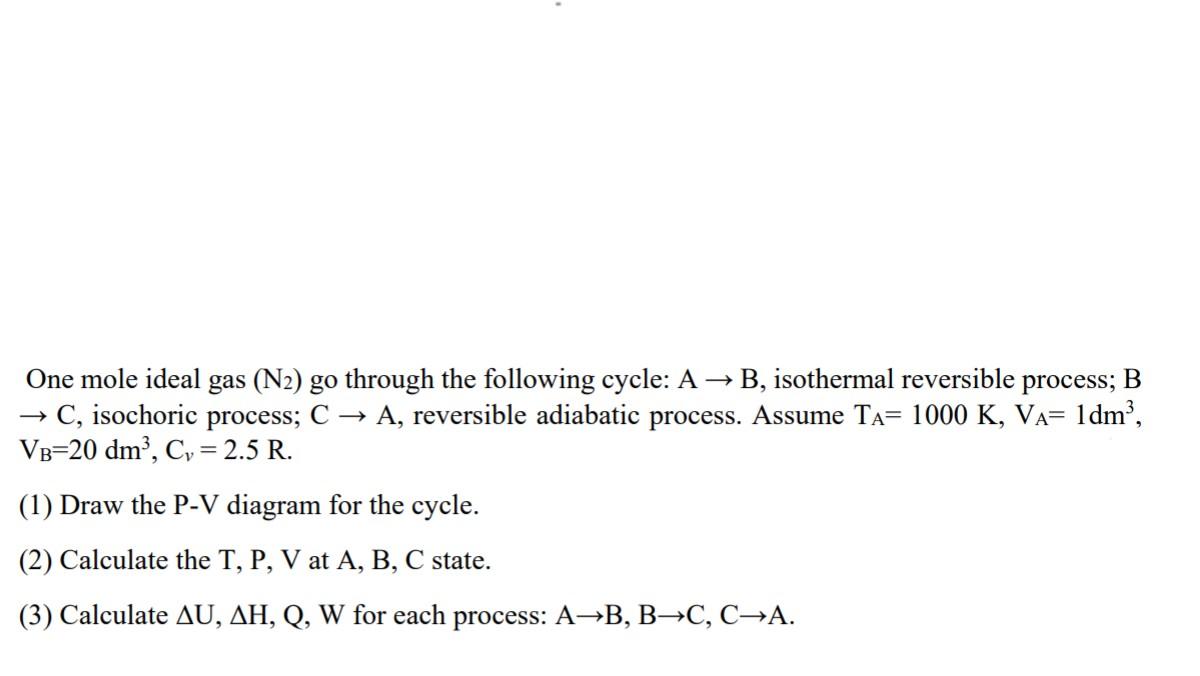
A constant volume gas thermometer of volume 1x10-3 m3 contains 0.05 mol of gas. It is assumed that the gas obeys the perfect gas law, PV = RT ; but in fact, a its behaviour is better described by the equation P+- (V-b)= RT , 2 V. m m where a=0.8 Nm*mol-2 and b=3x10-5 m mol. The thermometer is calibrated at the triple point of water. What is the temperature reading at 100C, and what is the percentage error of the meter at 100C? One mole ideal gas (N2) go through the following cycle: A B, isothermal reversible process; B C, isochoric process; C A, reversible adiabatic process. Assume TA= 1000 K, VA= 1dm2, VB=20 dm', C, = 2.5 R. (1) Draw the P-V diagram for the cycle. (2) Calculate the T, P, V at A, B, C state. (3) Calculate AU, AH, Q, W for each process: AB, BC, CA.
Step by Step Solution
★★★★★
3.49 Rating (166 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


