Answered step by step
Verified Expert Solution
Question
1 Approved Answer
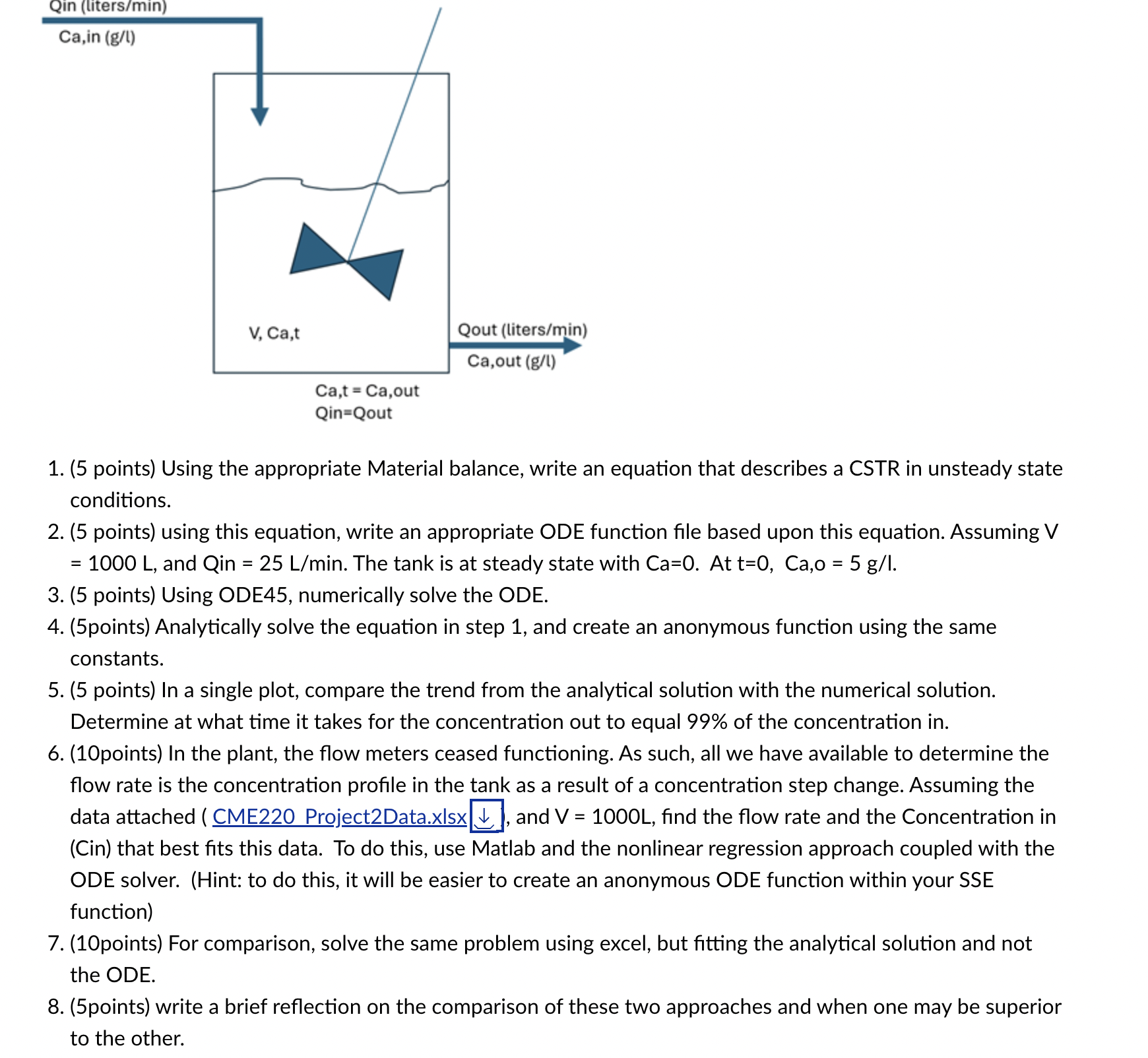
A Continuous Stirred - tank reactor ( CSTR ) is one of the classic conceptual models for a process reactor, that can be easily derived
A Continuous Stirredtank reactor CSTR is one of the classic conceptual models for a process reactor, that can be easily derived from applying a material balance, but keeping the unsteady state portion of the equation. In the simplest embodiment, you have an isothermal system with a single stream in and a single stream out. We assume that the mixing within the tank is so effective, fast and efficient, that the concentration of the reactant leaving the tank equals the concentration of the reactant in the tank. If there is no reaction in the tank and there is a change in concentration of compound A Cain in the tank feed, there will be a lag in time before the concentration out of the tank will equal the concentration fed into the tank. points Using the appropriate Material balance, write an equation that describes a CSTR in unsteady state
conditions.
points using this equation, write an appropriate ODE function file based upon this equation. Assuming
and Qin The tank is at steady state with Ca At t Cao
points Using ODE numerically solve the ODE.
points Analytically solve the equation in step and create an anonymous function using the same
constants.
points In a single plot, compare the trend from the analytical solution with the numerical solution.
Determine at what time it takes for the concentration out to equal of the concentration in
points In the plant, the flow meters ceased functioning. As such, all we have available to determine the
flow rate is the concentration profile in the tank as a result of a concentration step change. Assuming the
data attached CME ProjectData.xIsx darr, and V L find the flow rate and the Concentration in
Cin that best fits this data. To do this, use Matlab and the nonlinear regression approach coupled with the
ODE solver. Hint: to do this, it will be easier to create an anonymous ODE function within your SSE
function
points For comparison, solve the same problem using excel, but fitting the analytical solution and not
the ODE.
points write a brief reflection on the comparison of these two approaches and when one may be superior
to the other.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


