Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A convenient laboratory technique for measuring the kinetics of ideal gas phase single reactions is to follow the change in total pressure in a constant
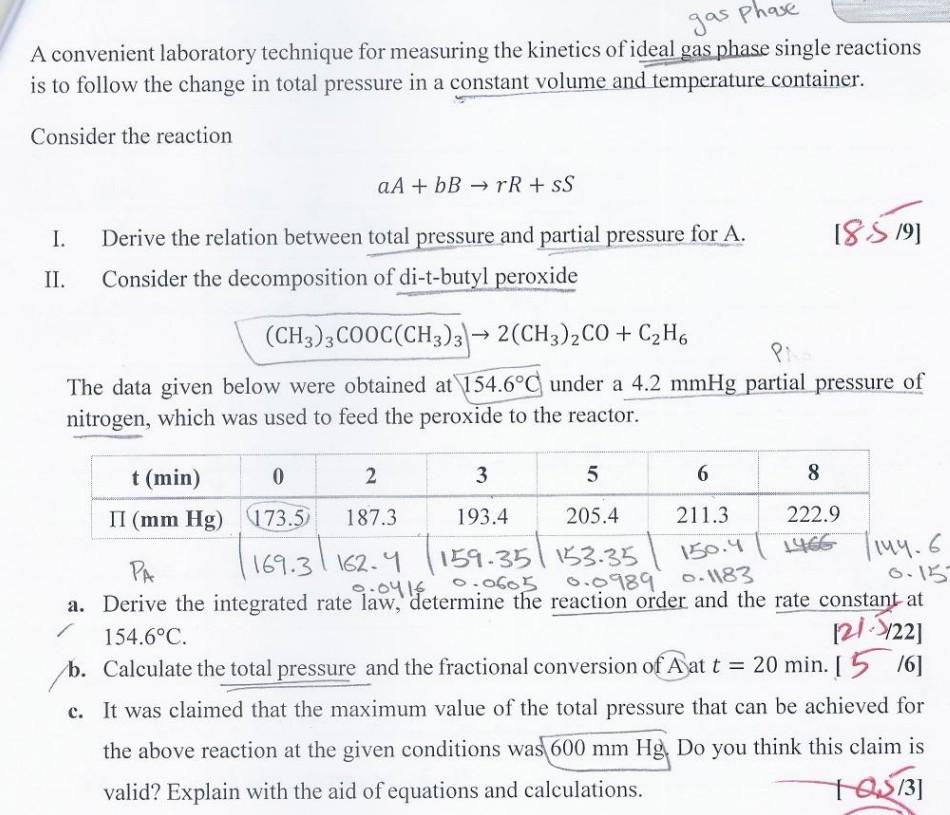
A convenient laboratory technique for measuring the kinetics of ideal gas phase single reactions is to follow the change in total pressure in a constant volume and temperature container. Consider the reaction aA+bBrR+sS I. Derive the relation between total pressure and partial pressure for A. [8:5/9] II. Consider the decomposition of di-t-butyl peroxide (CH3)3COOC(CH3)3)2(CH3)2CO+C2H6 The data given below were obtained at 154.6C under a 4.2mmHg partial pressure of nitrogen, which was used to feed the peroxide to the reactor. a. Derive the integrated rate law, determine the reaction order and the rate constant at 154.6C. [21.5/22] b. Calculate the total pressure and the fractional conversion of A at t=20min. [ 5/6] c. It was claimed that the maximum value of the total pressure that can be achieved for the above reaction at the given conditions was 600mmHg. Do you think this claim is valid? Explain with the aid of equations and calculations
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


