Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A Conver The reaction of NO(g) + Cl(g) NOCK(g) + CKg) is found to have an equilibrium constant of 1.1x10 at a particular temperature.
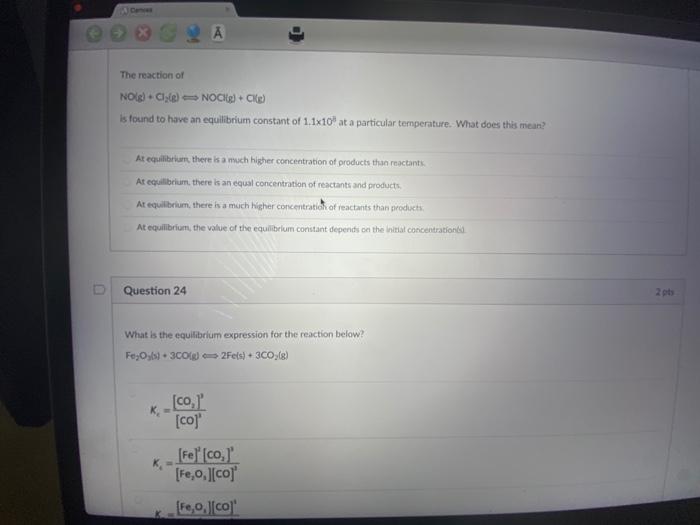
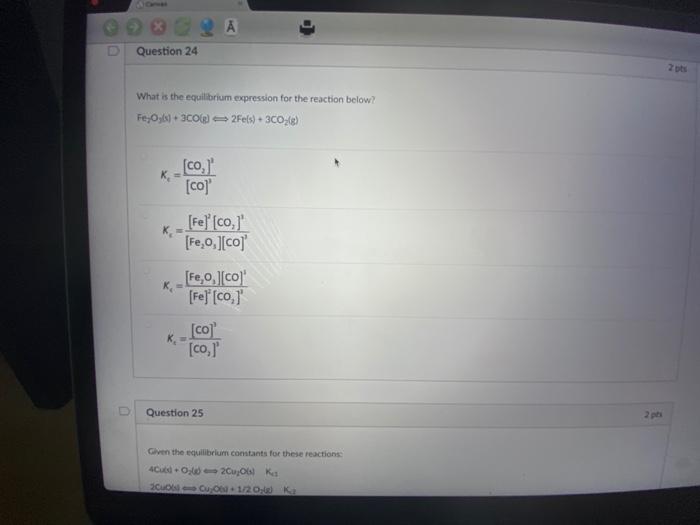
A Conver The reaction of NO(g) + Cl(g) NOCK(g) + CKg) is found to have an equilibrium constant of 1.1x10 at a particular temperature. What does this mean? 2 A At equilibrium, there is a much higher concentration of products than reactants At equilibrium, there is an equal concentration of reactants and products. At equilibrium, there is a much higher concentration of reactants than products. At equilibrium, the value of the equilibrium constant depends on the initial concentration D Question 24 What is the equilibrium expression for the reaction below? FeOys) + 3C0(g) 2Fe(s) + 3CO; (g) K K [co, [co]' [Fe] [co, [Fe,o,][co] [Fe,0,][co] 2 pts Question 24 What is the equilibrium expression for the reaction below? Fe,Oys) + 3C0(g) 2Fe(s) + 3CO(g) _[co,]' [co]' [Fe] [co,]' [FeO,][co] K [Fe,o,][co]' [Fe] [co,]' [co] [co,]' Question 25 Given the equilibrium constants for these reactions: 2Cu06) Kes 4Cubd-O 20u06) Cu 06-1/20g) Ka 2 pts 2 pts
Step by Step Solution
★★★★★
3.43 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1
Question23 Solution The given chemical reaction is as follows NO g Cl 2 g NOCl g Cl g The value of t...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


