Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The following sequence of reactions was used to synthesize compound D. In all steps, the product of the preceding step is used in the
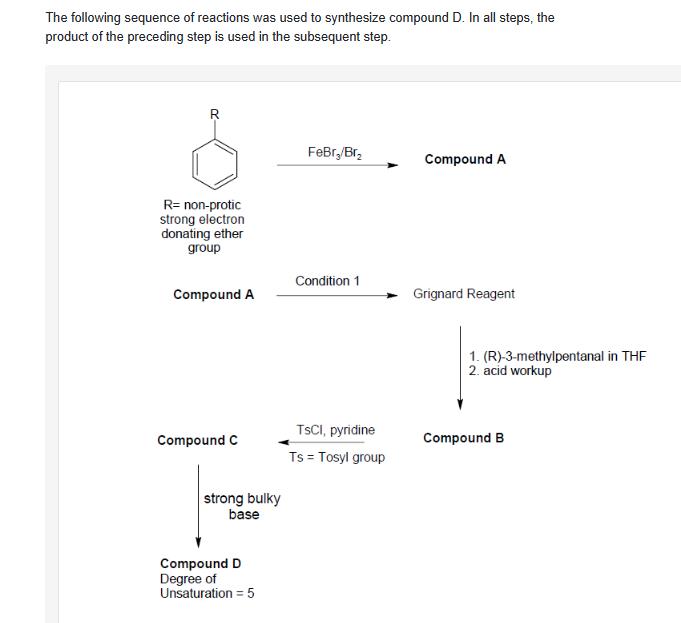

The following sequence of reactions was used to synthesize compound D. In all steps, the product of the preceding step is used in the subsequent step. R R= non-protic strong electron donating ether group FeBr/Br Compound A Condition 1 Compound A Grignard Reagent 1. (R)-3-methylpentanal in THF 2. acid workup TsCI, pyridine Compound C Compound B Ts = Tosyl group strong bulky base Compound D Degree of Unsaturation = 5 i)For each of the reactions in the above synthetic scheme, draw the major product (compounds A-D) of the reaction. Show all possible stereoisomers of compounds B and assign R/S nomenclature where relevant. Correctly identify the relationship(s) between the pair(s) of stereoisomer(s). ii) Write down the reaction conditions for 1 highlighting the actions to be taken for efficient formation of Grignard reagent. iii) Draw the accepted mechanism for the conversion of Compound C to D. Comment on the stereochemistry of the product. iv) Suggest the peaks expected in the region from 5-9 ppm on the 1H NMR spectrum for compound D, showing approx. chemical shift region, multiplicity, number of hydrogens and the corresponding partial structure.
Step by Step Solution
★★★★★
3.38 Rating (167 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


