Question
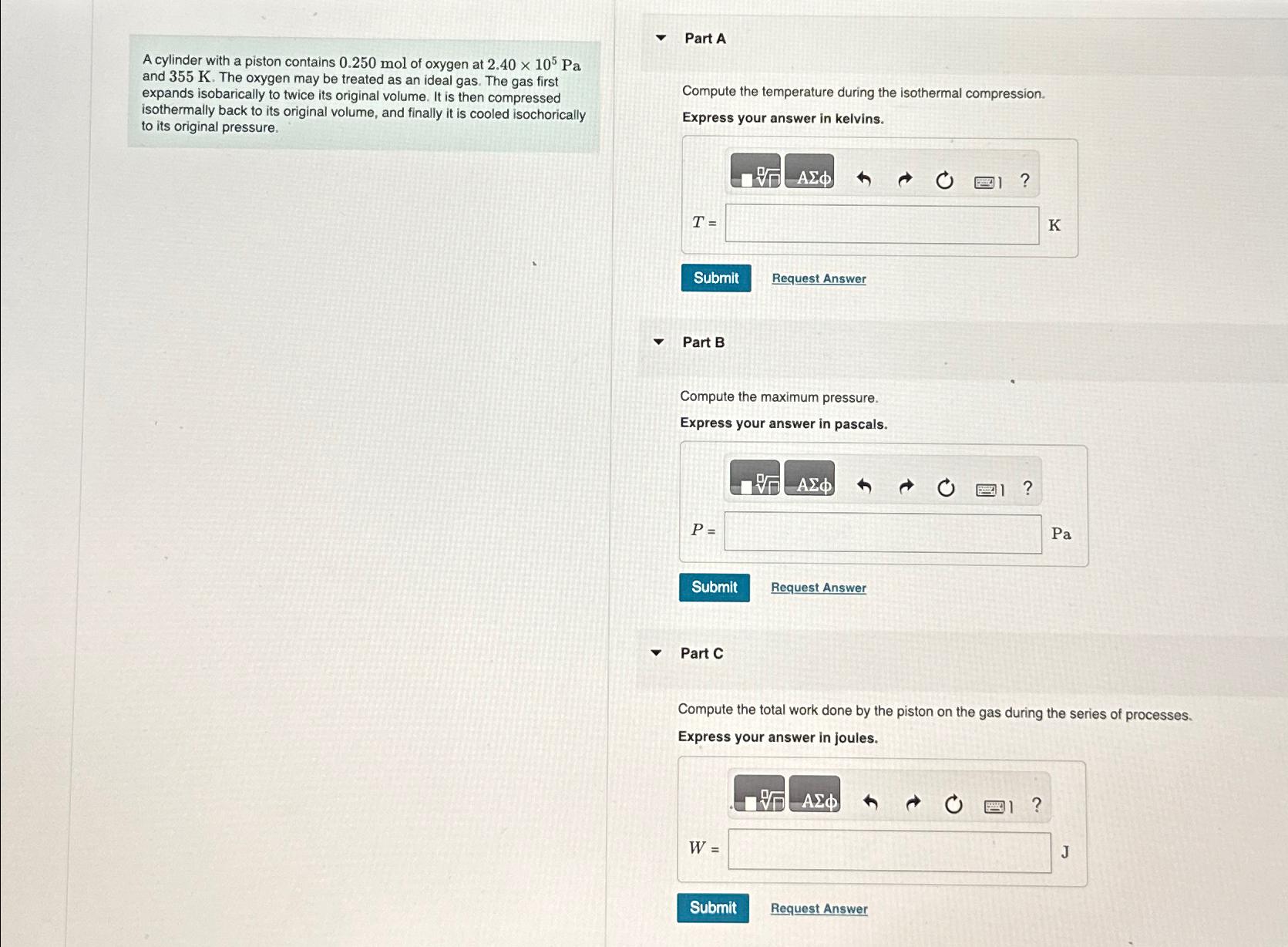
A cylinder with a piston contains 0.250mol of oxygen at 2.40times 10^(5)Pa and 355K . The oxygen may be treated as an ideal gas. The
A cylinder with a piston contains
0.250molof oxygen at
2.40\\\\times 10^(5)Paand
355K. The oxygen may be treated as an ideal gas. The gas first expands isobarically to twice its original volume. It is then compressed isothermally back to its original volume, and finally it is cooled isochorically to its original pressure.\ Part A\ Compute the temperature during the isothermal compression.\ Express your answer in kelvins.\ Request Answer\ Part B\ Compute the maximum pressure.\ Express your answer in pascals.\
P=\
Pa\ Request Answer\ Part C\ Compute the total work done by the piston on the gas during the series of processes.\ Express your answer in joules.\
W=\ Panc\ the piston on the during the series of processes.\ Express your answer in joules.\ Request Answer
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


