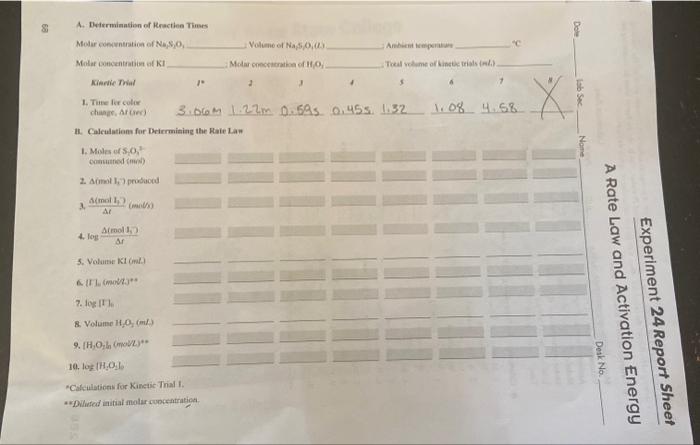
A. Deftrmianatien of Rractien Times Medar conventation of Nejsio Voliene of Na 5,0,,.j Amberit kenponias 4c Moler ionicentration ut KI Mctar creccitration of H1O. Ticeal volume of kinete trials (adit. kiarice Thial 1. Tute Inr culie chaser. Ar iner) 3.06m 1.2. 0.69m. 0.45s. 1.32 1.08 4.58 "Cateulaticen for Kanetic Trial 1. * Dlised zaitial molur ropcentratica. - To determine the rate law for a chemical reaction - To vtilize a graphical analysir of experimental data to - determine the order of each reactant in the reaction -determine the activation energy for the reaction The followine techniques are used in the Experimental Precedure: TECHNIQUES The rate of a chemical reaction is affectod by a number of factors, moie of which were oberved in Experiment 23. The tale of a reactioe can be expressed in a number of ways, depending on the nature of the reactants being consumet or the produces being forned. The rate may be followed as a change in concentration (mov2) of one of the tractants of products per unit of time, the volume of gas produeed per unit of time (figes 24.1), or the change in color (measured as light ahsorbanoe) per unit of time, jact to cite a few eumples. In Parts A-D of this experiment, a quantiative statement is determined as to how ehanges in reactant concentrations affect reaction rate at room temperature, the saisment being the rate law for the reaction. In Part B, the reaction rale is detcmined at different temperasurts, allowing os to use the data to calculate the activation conersy fos the Figure 24.1 The rate of reaction. To assist in underntanding the relationship between reactant conccotration and tharmal deconposition of coleium reaction rate, consider the general reactioa, A2+2B22AB2. The fate of this reas- carbonate is delermined by tion is related, by some exponential power, to the initial cancentration of each reactats. megasing ithe volume of ovolved For this reaction, we can write the relationship as rate=k(A2)(B2)r (24.1) This expression is called the rate law for the reaction. The value of i, the reaction rate constant, varies with temperature bat is isdependent of reactant eoncentritions. Rale conslont a proporitionelily The superiseripts p and q designale the order with respect to cach reactant and are constant calating the rute of a always determined experimentally. For example, if tripling the molar concentratige of of the mociant A2 while bolding the B2 conceatration constast increases the rexction rate by a factor of 9 , then =2. In practice, when the B2 concentration is in large excess relative to Ordes the exponentiol foctor by. the Ay concentration, the By concentritiod remains essentially constumt during the which the concentrotion of a. course of the reaction; therefore, the change in the reaction rate recoles from the more: significant change in the imaller amount of A2 in the reaction. An experimental stady. of the kinetics of any reaction involves detersining the valiess of k in and q. Experiment 24281 Table 24.1 Composition of Test Solufions 5. Repeat for the rcmainiag Kinctic triaks Mix atd lime the asu wolvions for the remaining ieven kincoe trialk. If the in itructio appones, ocedoct asditsenal kinctic uials, eithes by Jepeating thoie in Tahin 24 T or by preparing ceher combinarioes of KI and H2. Make mue thas the local diluted vohane remaits constate at 10mL Diepotale Dispose of the solutions from the Linetic tricls in the Watle lod de Selts containor. CLEANUP: Rinse the bealen of test fultes twise with typ mater and diveard in the Waste lodide Salts comtainer. Dispove of two finul riases with diciceived wuer ia the tiak. Perform the ealculutions, earefully one itep ar e hime. Apoopriate asd comoctly pro- H. Cale blations for gramamed software would be invalable for coepletits thiy amalyi. Asiyee read Determialing the Rate Law through this section, coumplest the appoprice caledhibe asd rocord it with the cerrect number of eignificas fieuec for each seit wolutice oe the Roping Sluef. 1. Moles of 33 produced. Calcalate the moles of S2O32. cobsenod is each Linctuc Irial. From equation 24.13, the molos of , that fons in the reactice cysals coeikaning with noine at time rero up unid a final unouint that was prodeced at the lime of the color change. This is designeted a. -Aitaod 1,7" prodaced See Prrlab- orutay Arignment, gueation 5.B:1,2. 2. Reaction rate. The rearmon rade for each kinictis thial is calcullatod as the rasio of sthem on the Repart. Shrer. See Prelaberatagy Axiremong, qaestice 5a,1,4, 4 Becaine the toial vedune at a comatait for alf Lisntic trials, we nely beed le calsty 1. Initial bodide concentrations. Calcuiale sho iaitial moitur quecentrices. It he and the logaritim of the initial motar conoenaratint las It lis of bolife isn for each. A. Deftrmianatien of Rractien Times Medar conventation of Nejsio Voliene of Na 5,0,,.j Amberit kenponias 4c Moler ionicentration ut KI Mctar creccitration of H1O. Ticeal volume of kinete trials (adit. kiarice Thial 1. Tute Inr culie chaser. Ar iner) 3.06m 1.2. 0.69m. 0.45s. 1.32 1.08 4.58 "Cateulaticen for Kanetic Trial 1. * Dlised zaitial molur ropcentratica. - To determine the rate law for a chemical reaction - To vtilize a graphical analysir of experimental data to - determine the order of each reactant in the reaction -determine the activation energy for the reaction The followine techniques are used in the Experimental Precedure: TECHNIQUES The rate of a chemical reaction is affectod by a number of factors, moie of which were oberved in Experiment 23. The tale of a reactioe can be expressed in a number of ways, depending on the nature of the reactants being consumet or the produces being forned. The rate may be followed as a change in concentration (mov2) of one of the tractants of products per unit of time, the volume of gas produeed per unit of time (figes 24.1), or the change in color (measured as light ahsorbanoe) per unit of time, jact to cite a few eumples. In Parts A-D of this experiment, a quantiative statement is determined as to how ehanges in reactant concentrations affect reaction rate at room temperature, the saisment being the rate law for the reaction. In Part B, the reaction rale is detcmined at different temperasurts, allowing os to use the data to calculate the activation conersy fos the Figure 24.1 The rate of reaction. To assist in underntanding the relationship between reactant conccotration and tharmal deconposition of coleium reaction rate, consider the general reactioa, A2+2B22AB2. The fate of this reas- carbonate is delermined by tion is related, by some exponential power, to the initial cancentration of each reactats. megasing ithe volume of ovolved For this reaction, we can write the relationship as rate=k(A2)(B2)r (24.1) This expression is called the rate law for the reaction. The value of i, the reaction rate constant, varies with temperature bat is isdependent of reactant eoncentritions. Rale conslont a proporitionelily The superiseripts p and q designale the order with respect to cach reactant and are constant calating the rute of a always determined experimentally. For example, if tripling the molar concentratige of of the mociant A2 while bolding the B2 conceatration constast increases the rexction rate by a factor of 9 , then =2. In practice, when the B2 concentration is in large excess relative to Ordes the exponentiol foctor by. the Ay concentration, the By concentritiod remains essentially constumt during the which the concentrotion of a. course of the reaction; therefore, the change in the reaction rate recoles from the more: significant change in the imaller amount of A2 in the reaction. An experimental stady. of the kinetics of any reaction involves detersining the valiess of k in and q. Experiment 24281 Table 24.1 Composition of Test Solufions 5. Repeat for the rcmainiag Kinctic triaks Mix atd lime the asu wolvions for the remaining ieven kincoe trialk. If the in itructio appones, ocedoct asditsenal kinctic uials, eithes by Jepeating thoie in Tahin 24 T or by preparing ceher combinarioes of KI and H2. Make mue thas the local diluted vohane remaits constate at 10mL Diepotale Dispose of the solutions from the Linetic tricls in the Watle lod de Selts containor. CLEANUP: Rinse the bealen of test fultes twise with typ mater and diveard in the Waste lodide Salts comtainer. Dispove of two finul riases with diciceived wuer ia the tiak. Perform the ealculutions, earefully one itep ar e hime. Apoopriate asd comoctly pro- H. Cale blations for gramamed software would be invalable for coepletits thiy amalyi. Asiyee read Determialing the Rate Law through this section, coumplest the appoprice caledhibe asd rocord it with the cerrect number of eignificas fieuec for each seit wolutice oe the Roping Sluef. 1. Moles of 33 produced. Calcalate the moles of S2O32. cobsenod is each Linctuc Irial. From equation 24.13, the molos of , that fons in the reactice cysals coeikaning with noine at time rero up unid a final unouint that was prodeced at the lime of the color change. This is designeted a. -Aitaod 1,7" prodaced See Prrlab- orutay Arignment, gueation 5.B:1,2. 2. Reaction rate. The rearmon rade for each kinictis thial is calcullatod as the rasio of sthem on the Repart. Shrer. See Prelaberatagy Axiremong, qaestice 5a,1,4, 4 Becaine the toial vedune at a comatait for alf Lisntic trials, we nely beed le calsty 1. Initial bodide concentrations. Calcuiale sho iaitial moitur quecentrices. It he and the logaritim of the initial motar conoenaratint las It lis of bolife isn for each