Answered step by step
Verified Expert Solution
Question
1 Approved Answer
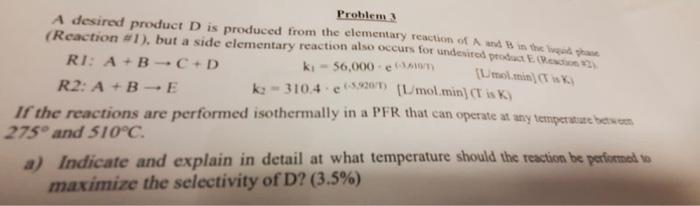
A desired product D is produced from the elementary reaction of A and B in the liquid ghase (Reaction #I), but a side elementary reaction
A desired product D is produced from the elementary reaction of A and B in the liquid ghase (Reaction #I), but a side elementary reaction also occurs for undesired product E. (Reaction #7.
RI: A + B C+ D
ki = 56,000 - e (-3,610/1)
R2: A + B - E
(L mol.min) (T is K)
ka - 310.4 - c (5.9307) (L/mol min) (T is K)
If the reactions are performed isothermally in a PFR that can operate at any temperature between
275 and 510C.
a)
Indicate and explain in detail at what temperature should the reaction be performed to maximize the selectivity of D? (3.5%)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


