Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A piston-cylinder assembly contains I mol of gaseous ethyl alcohol (CH,OH) and 3.5 mol of oxygen (O) initially at 25 C and I atm.
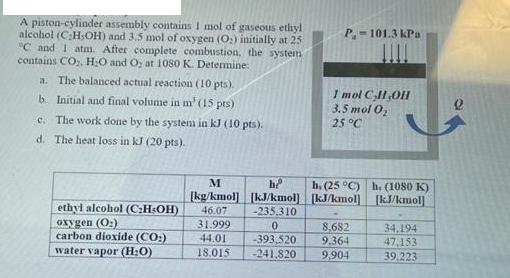
A piston-cylinder assembly contains I mol of gaseous ethyl alcohol (CH,OH) and 3.5 mol of oxygen (O) initially at 25 "C and I atm. After complete combustion, the system contains CO, HO and Oy at 1080 K. Determine: a. The balanced actual reaction (10 pts). b. Initial and final volume in m' (15 pts) c. The work done by the system in kJ (10 pts). d. The heat loss in kJ (20 pts). ethyl alcohol (CH-OH) oxygen (O) carbon dioxide (CO:) water vapor (H:O) P.-101.3 kPa -393,520 -241.820 1 mol C,H,OH 3.5 mol O 25 C M h [kg/kmol] [kJ/kmol] [kJ/kmol] 46.07 -235,310 31.999 0 44.01 18.015 h. (25 C) h. (1080 K) [kJ/kmol 8.682 9.364 9,904 34,194 47,153 39.223 2
Step by Step Solution
★★★★★
3.46 Rating (149 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


