Answered step by step
Verified Expert Solution
Question
1 Approved Answer
(a) Determine the fugacity and fugacity coefficient for isobutane at 40C(313.15K) and pressure of 2 bar, 5.28 bar and 8 bar by using the given
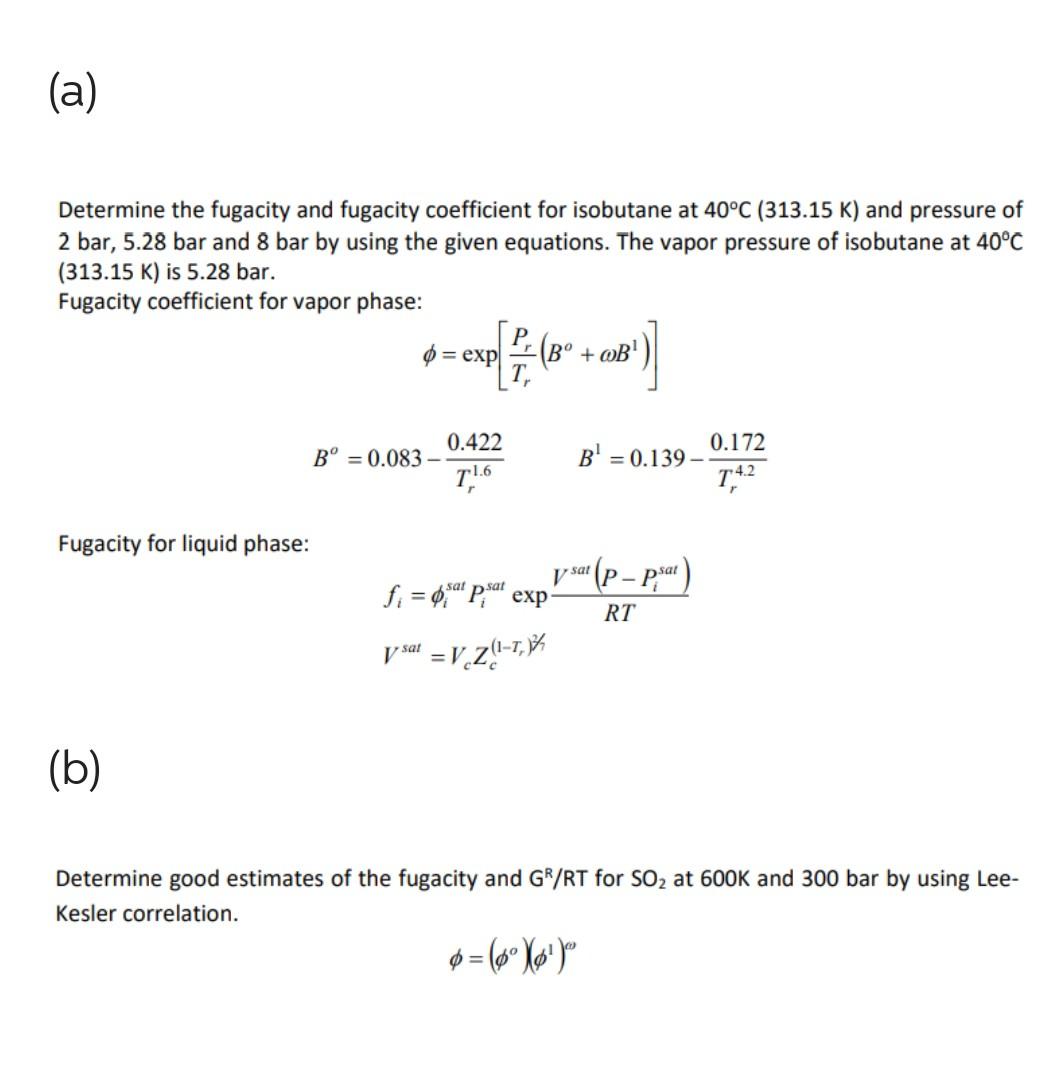
(a) Determine the fugacity and fugacity coefficient for isobutane at 40C(313.15K) and pressure of 2 bar, 5.28 bar and 8 bar by using the given equations. The vapor pressure of isobutane at 40C (313.15K) is 5.28 bar. Fugacity coefficient for vapor phase: =exp[TrPr(Bo+B1)]Bo=0.083Tr1.60.422B1=0.139Tr4.20.172 Fugacity for liquid phase: fi=isatPisatexpRTVsat(PPisat)Vsat=VcZc(1Tr)2/2 (b) Determine good estimates of the fugacity and GR/RT for SO2 at 600K and 300 bar by using LeeKesler correlation. =(o)(1)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


