Question
A. Draw a labeled, schematic of the process, indicating all inlet/outlet streams and conditions B. Determine the value of the equilibrium constant at the conditions
A. Draw a labeled, schematic of the process, indicating all inlet/outlet streams and conditions
B. Determine the value of the equilibrium constant at the conditions of the reactor. If you are unable to obtain an answer for this part and require one for subsequent parts, presume that K = 10.
C. Write expressions for the composition of the reactor components (SO2, SO3, O2, and N2) using a basis of 1 mol SO2. Using any reasonable or necessary approximations, obtain an expression for the equilibrium constant as a function of the extent of reaction ξ. You do not need a numerical result.
D. Determine the amount of heat (per mole of SO2 fed to the reactor) that must be added or removed from the reactor to maintain isothermal operation. If you do not know the extent of reaction, presume that the reaction proceeds to equilibrium.
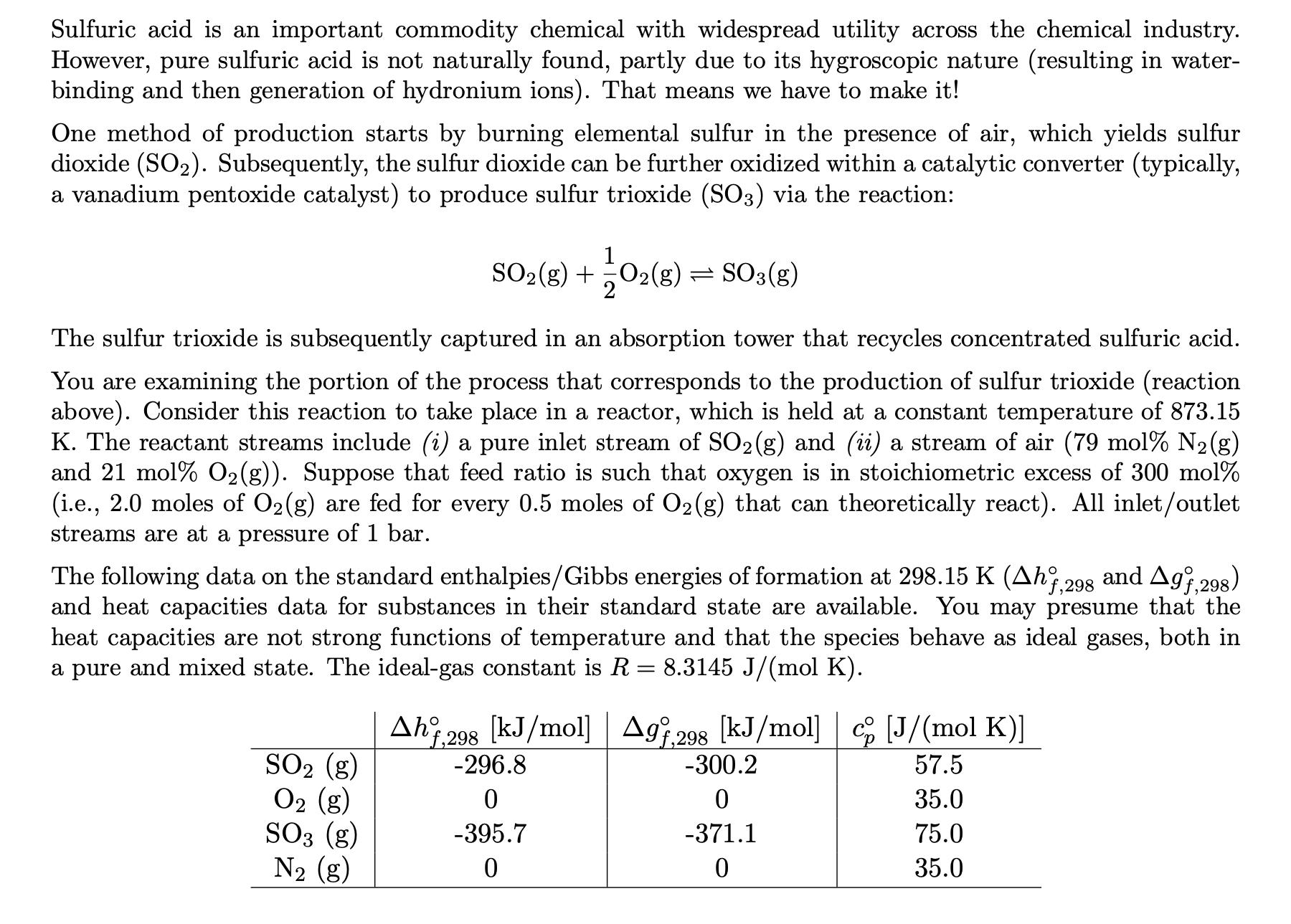
Sulfuric acid is an important commodity chemical with widespread utility across the chemical industry. However, pure sulfuric acid is not naturally found, partly due to its hygroscopic nature (resulting in water- binding and then generation of hydronium ions). That means we have to make it! One method of production starts by burning elemental sulfur in the presence of air, which yields sulfur dioxide (SO). Subsequently, the sulfur dioxide can be further oxidized within a catalytic converter (typically, a vanadium pentoxide catalyst) to produce sulfur trioxide (SO3) via the reaction: The sulfur trioxide is subsequently captured in an absorption tower that recycles concentrated sulfuric acid. You are examining the portion of the process that corresponds to the production of sulfur trioxide (reaction above). Consider this reaction to take place in a reactor, which is held at a constant temperature of 873.15 K. The reactant streams include (i) a pure inlet stream of SO2(g) and (ii) a stream of air (79 mol% N(g) and 21 mol % O(g)). Suppose that feed ratio is such that oxygen is in stoichiometric excess of 300 mol% (i.e., 2.0 moles of O(g) are fed for every 0.5 moles of O(g) that can theoretically react). All inlet/outlet streams are at a pressure of 1 bar. 1 SO(g) + O2(g) SO3(g) = 2020 and Agf,298) The following data on the standard enthalpies/Gibbs energies of formation at 298.15 K (Ahf,298 and heat capacities data for substances in their standard state are available. You may presume that the heat capacities are not strong functions of temperature and that the species behave as ideal gases, both in a pure and mixed state. The ideal-gas constant is R = 8.3145 J/(mol K). SO (g) O (g) SO3 (g) N (g) Ahf 298 [kJ/mol] | Agf,298 [kJ/mol] c [J/(mol K)] | 57.5 35.0 75.0 35.0 -296.8 0 -395.7 0 -300.2 0 -371.1 0
Step by Step Solution
3.48 Rating (161 Votes )
There are 3 Steps involved in it
Step: 1
A Sch...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


