Answered step by step
Verified Expert Solution
Question
1 Approved Answer
a. Estimate the heat and work flows needed to reversibly and isothermally separate an equimolar mixture of two species into its pure components if the
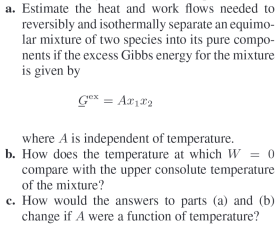 a. Estimate the heat and work flows needed to reversibly and isothermally separate an equimolar mixture of two species into its pure components if the excess Gibbs energy for the mixture is given by Gex=Ax1x2 Where A is independent of temperature. b. How does the temperature at which W=0 compare with the upper consolute temperature of the mixture? c. How would the answers to parts (a) and (b) change if A were a function of temperature
a. Estimate the heat and work flows needed to reversibly and isothermally separate an equimolar mixture of two species into its pure components if the excess Gibbs energy for the mixture is given by Gex=Ax1x2 Where A is independent of temperature. b. How does the temperature at which W=0 compare with the upper consolute temperature of the mixture? c. How would the answers to parts (a) and (b) change if A were a function of temperature Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


