Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Calculate the mass fraction stochiometric ratio for the combustion of Ethane (CHs) in air (20% v/v Oz 80% v/v N2) Given the following Atomic
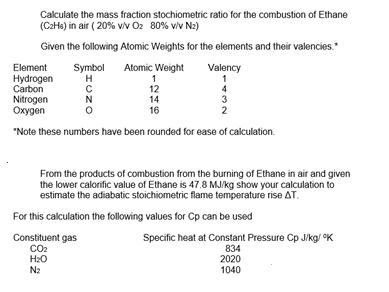
Calculate the mass fraction stochiometric ratio for the combustion of Ethane (CHs) in air (20% v/v Oz 80% v/v N2) Given the following Atomic Weights for the elements and their valencies." Symbol Atomic Weight H Element Hydrogen Carbon Nitrogen Oxygen *Note these numbers have been rounded for ease of calculation. N 1 12 14 16 Valency 1 4 3 2 From the products of combustion from the burning of Ethane in air and given the lower calorific value of Ethane is 47.8 MJ/kg show your calculation to estimate the adiabatic stoichiometric flame temperature rise AT. For this calculation the following values for Cp can be used Constituent gas CO HO N Specific heat at Constant Pressure Cp J/kg/ K 834 2020 1040
Step by Step Solution
★★★★★
3.36 Rating (162 Votes )
There are 3 Steps involved in it
Step: 1
To calculate the mass fraction stoichiometric ratio for the combustion of Ethane C2H5 in air 20 vv O2 and 80 vv N2 and estimate the adiabatic stoichio...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


