Question: Isopropyl ether (E) is used to separate acetic acid (A) from water (W). The liquid-liquid equilibrium data at 25oC and 1 atm(a) One hundred kilograms
Isopropyl ether (E) is used to separate acetic acid (A) from water (W). The liquid-liquid equilibrium data at 25oC and 1 atm(a) One hundred kilograms of a 30 wt% A-W solution is contacted with 120 kg of ether in an equilibrium stage. What are the compositions and weights of the resulting extract and raffinate? What would be the concentration of acid in the (ether-rich) extract if all the ether were removed?(b) A mixture containing 52 kg A and 48 kg W is contacted with 40 kg of E. What are the extract and raffinate compositions andquantities?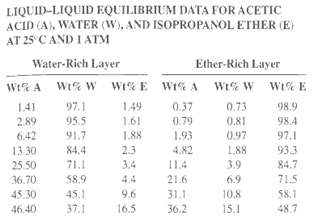
LIQUID-LIQUID EQUILIBRIUM DATA FOR ACETIC ACID (A), WATER (W), AND ISOPROPANOL ETHER (E) AT 25 CAND I ATM Water-Rich Layer Ether-Rich Layer WiG A Wt% W Wi% E WI% A Wt% W wt% E 141 2.89 97.1 1.49 0.37 0.73 98.9 95.5 1.61 0.79 0.81 0.97 98.4 6.42 91.7 1.88 1.93 4,82 97.1 2.3 13.30 84.4 1,88 933 71.1 3.4 11.4 3.9 84.7 25.50 71.5 36.70 58.9 4.4 21.6 6.9 31.1 45.30 45.1 9.6 10.8 $8.1 16.5 46.40 37.1 15.1 48,7 36.2
Step by Step Solution
3.39 Rating (161 Votes )
There are 3 Steps involved in it
Use a righttriangle diagram of the equilibrium data which is easily produced with a spreadsheet a In ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
37-E-C-E-S-P (138).docx
120 KBs Word File


