Question: A countercurrent system with N equilibrium stages is used to separate acetic acid from water in a ternary system of water - acetic acid -
A countercurrent system with N equilibrium stages is used to separate acetic acid from water in
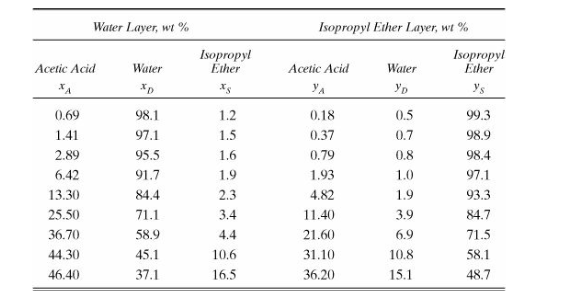
a ternary system of wateracetic acidisopropyl ether as shown below. Feed is wt acetic acid and wt water. Feed flow rate is
kgh Solvent is a pure isopropyl ether with a flow rate S of times larger than the minimum required solvent flow rate. The extraction process should decrease the acetic acid concentration in the outlet raffinate stream R to wt xAFind the minimum solvent flow rate SminAfter finding the real solvent flow rate S find the flow rate and concentration of the streams R xA xD EN yAN yDN Data to make the diagram is attached
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


