Question: Isopropyl ether (E) is used to separate acetic acid (A) from water (W). The liquid-liquid equilibrium data at 25C and 1 atm are given below:
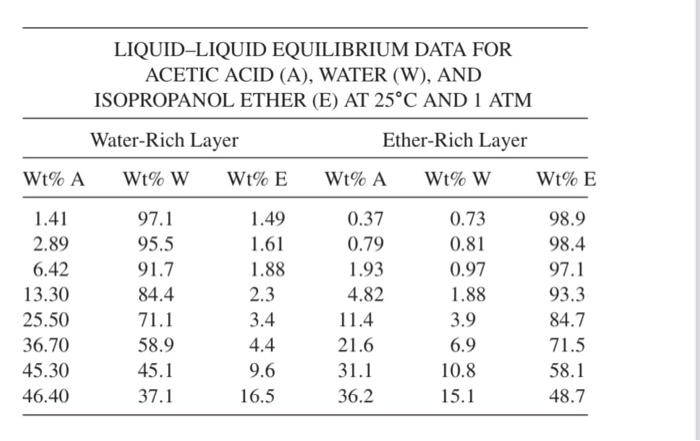

Isopropyl ether (E) is used to separate acetic acid (A) from water (W). The liquid-liquid equilibrium data at 25C and 1 atm are given below: (a) One hundred kilograms of a 30 wt% A-W solution is contacted with 120 kg of ether (E). What are the compositions and weights of the resulting extract and raffinate? What would the concentration of acid in the (ether-rich) extract be if all ether were removed? (b) A solution of 52 kg A and 48 kg W is contacted with 40 kg of E. Calculate the extract and raffinate compositions and quantities. LIQUID-LIQUID EQUILIBRIUM DATA FOR ACETIC ACID (A), WATER (W), AND ISOPROPANOL ETHER (E) AT 25C AND 1 ATM Water-Rich Layer Ether-Rich Layer Wt%A Wt% W Wt% E Wt% A Wt% W Wt% E 1.41 2.89 6.42 13.30 25.50 36.70 45.30 46.40 97.1 95.5 91.7 84.4 71.1 58.9 45.1 37.1 1.49 1.61 1.88 2.3 3.4 4.4 9.6 16.5 0.37 0.79 1.93 4.82 11.4 21.6 31.1 36.2 0.73 0.81 0.97 1.88 3.9 6.9 10.8 15.1 98.9 98.4 97.1 93.3 84.7 71.5 58.1 48.7 See below for a "blank" triangle plot. You will need to plot the data from the problem on the figure: The data define tie lines and will allow you to draw the miscibility boundary. Waterproof CAM
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


