Answered step by step
Verified Expert Solution
Question
1 Approved Answer
(a) Following figure shows a schematio dingram of a column contactor for the extraction of a metal ion M2+ using a quatemary amine R4N+X2 dissolved
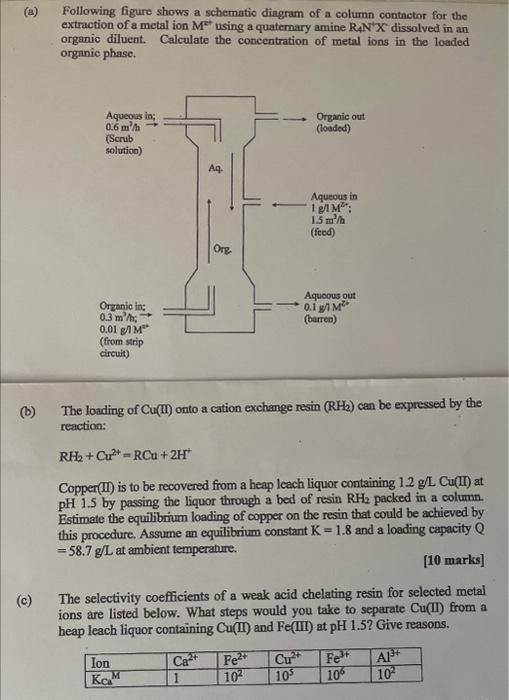 (a) Following figure shows a schematio dingram of a column contactor for the extraction of a metal ion M2+ using a quatemary amine R4N+X2 dissolved in an organic diluent. Calculate the concentration of metal ions in the loaded organie phase. (b) The loading of Cu(II) onto a cation exchange resin (RH2) can be expressed by the reaction: RH2+Cu2+=RCu+2H+ Copper(II) is to be recovered from a heap leach liquor containing 1.2g/LCu(II) at pH1.5 by passing the liquor through a bed of resin RH2 packed in a column. Estimate the equilibrium loading of copper on the resin that could be achieved by this procedure. Assume an equilibrium constant K=1.8 and a loading capacity Q =58.7g/L at ambient temperahure. [10 marks] (c) The selectivity coefficients of a weak acid chelating resin for selected metal ions are listed below. What steps would you take to separate Cu(II) from a heap leach liquor containing Cu( II) and Fe( III) at pH1.5 ? Give reasons
(a) Following figure shows a schematio dingram of a column contactor for the extraction of a metal ion M2+ using a quatemary amine R4N+X2 dissolved in an organic diluent. Calculate the concentration of metal ions in the loaded organie phase. (b) The loading of Cu(II) onto a cation exchange resin (RH2) can be expressed by the reaction: RH2+Cu2+=RCu+2H+ Copper(II) is to be recovered from a heap leach liquor containing 1.2g/LCu(II) at pH1.5 by passing the liquor through a bed of resin RH2 packed in a column. Estimate the equilibrium loading of copper on the resin that could be achieved by this procedure. Assume an equilibrium constant K=1.8 and a loading capacity Q =58.7g/L at ambient temperahure. [10 marks] (c) The selectivity coefficients of a weak acid chelating resin for selected metal ions are listed below. What steps would you take to separate Cu(II) from a heap leach liquor containing Cu( II) and Fe( III) at pH1.5 ? Give reasons

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


