Question
a) For a given undercooling AT, solid-liquid interfacial energy o, melting point TM. and latent heat L, can you express critical nucleus size for
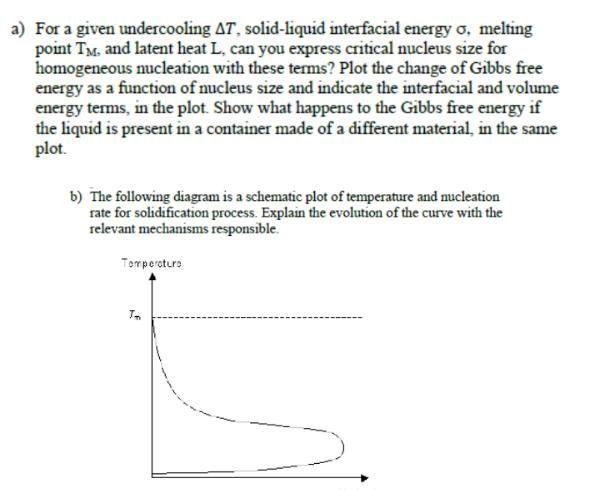
a) For a given undercooling AT, solid-liquid interfacial energy o, melting point TM. and latent heat L, can you express critical nucleus size for homogeneous nucleation with these terms? Plot the change of Gibbs free energy as a function of nucleus size and indicate the interfacial and volume energy terms, in the plot. Show what happens to the Gibbs free energy if the liquid is present in a container made of a different material, in the same plot. b) The following diagram is a schematic plot of temperature and nucleation rate for solidification process. Explain the evolution of the curve with the relevant mechanisms responsible. Temperature Tm
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Modern Classical Physics Optics Fluids Plasmas Elasticity Relativity And Statistical Physics
Authors: Kip S. Thorne, Roger D. Blandford
1st Edition
0691159025, 978-0691159027
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



