Question
How much heat in kJ is released when 20.3 g of NH3 is formed in the reaction shown below? N2(g) +3 H2(g) 2 NH3(g)
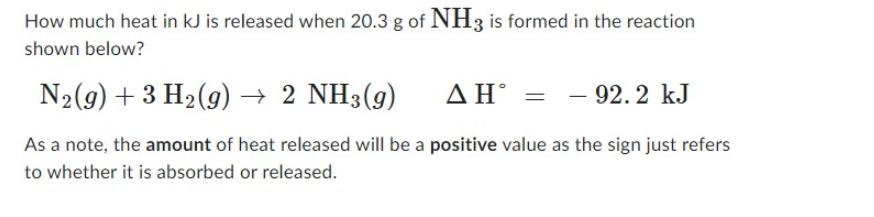
How much heat in kJ is released when 20.3 g of NH3 is formed in the reaction shown below? N2(g) +3 H2(g) 2 NH3(g) - 92.2 kJ As a note, the amount of heat released will be a positive value as the sign just refers to whether it is absorbed or released.
Step by Step Solution
There are 3 Steps involved in it
Step: 1
The given reaction is N2g 3 H2g 2 NH3g H 922 kJ The negative sign indicates that the reaction releas...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Biochemistry Concepts and Connections
Authors: Dean R. Appling, Spencer J. Anthony Cahill, Christopher K. Mathews
1st edition
321839927, 978-0133853490, 133853497, 978-0321839923
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



