Answered step by step
Verified Expert Solution
Question
1 Approved Answer
(a) For the solidification of iron, calculate the critical radius r and the activation free energy G if nucleation is homogeneous. Values for the latent
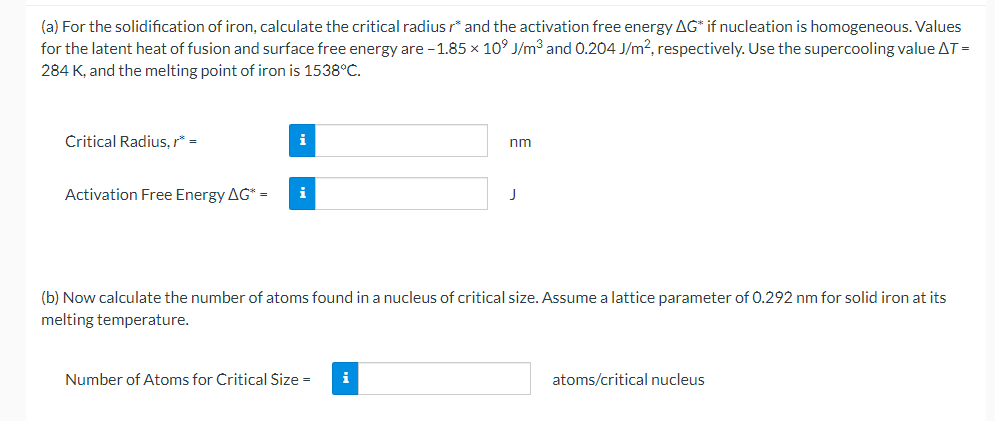 (a) For the solidification of iron, calculate the critical radius r and the activation free energy G if nucleation is homogeneous. Values for the latent heat of fusion and surface free energy are 1.85109J/m3 and 0.204J/m2, respectively. Use the supercooling value T= 284K, and the melting point of iron is 1538C. Critical Radius, r= Activation Free Energy G= (b) Now calculate the number of atoms found in a nucleus of critical size. Assume a lattice parameter of 0.292nm for solid iron at its melting temperature. Number of Atoms for Critical Size = atoms/critical nucleus (a) For the solidification of iron, calculate the critical radius r and the activation free energy G if nucleation is homogeneous. Values for the latent heat of fusion and surface free energy are 1.85109J/m3 and 0.204J/m2, respectively. Use the supercooling value T= 284K, and the melting point of iron is 1538C. Critical Radius, r= Activation Free Energy G= (b) Now calculate the number of atoms found in a nucleus of critical size. Assume a lattice parameter of 0.292nm for solid iron at its melting temperature. Number of Atoms for Critical Size = atoms/critical nucleus
(a) For the solidification of iron, calculate the critical radius r and the activation free energy G if nucleation is homogeneous. Values for the latent heat of fusion and surface free energy are 1.85109J/m3 and 0.204J/m2, respectively. Use the supercooling value T= 284K, and the melting point of iron is 1538C. Critical Radius, r= Activation Free Energy G= (b) Now calculate the number of atoms found in a nucleus of critical size. Assume a lattice parameter of 0.292nm for solid iron at its melting temperature. Number of Atoms for Critical Size = atoms/critical nucleus (a) For the solidification of iron, calculate the critical radius r and the activation free energy G if nucleation is homogeneous. Values for the latent heat of fusion and surface free energy are 1.85109J/m3 and 0.204J/m2, respectively. Use the supercooling value T= 284K, and the melting point of iron is 1538C. Critical Radius, r= Activation Free Energy G= (b) Now calculate the number of atoms found in a nucleus of critical size. Assume a lattice parameter of 0.292nm for solid iron at its melting temperature. Number of Atoms for Critical Size = atoms/critical nucleus Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


