Question
A gas contained in a piston-cylinder assembly undergoes two processes, A and B, between the same end states, 1 and 2, where p =
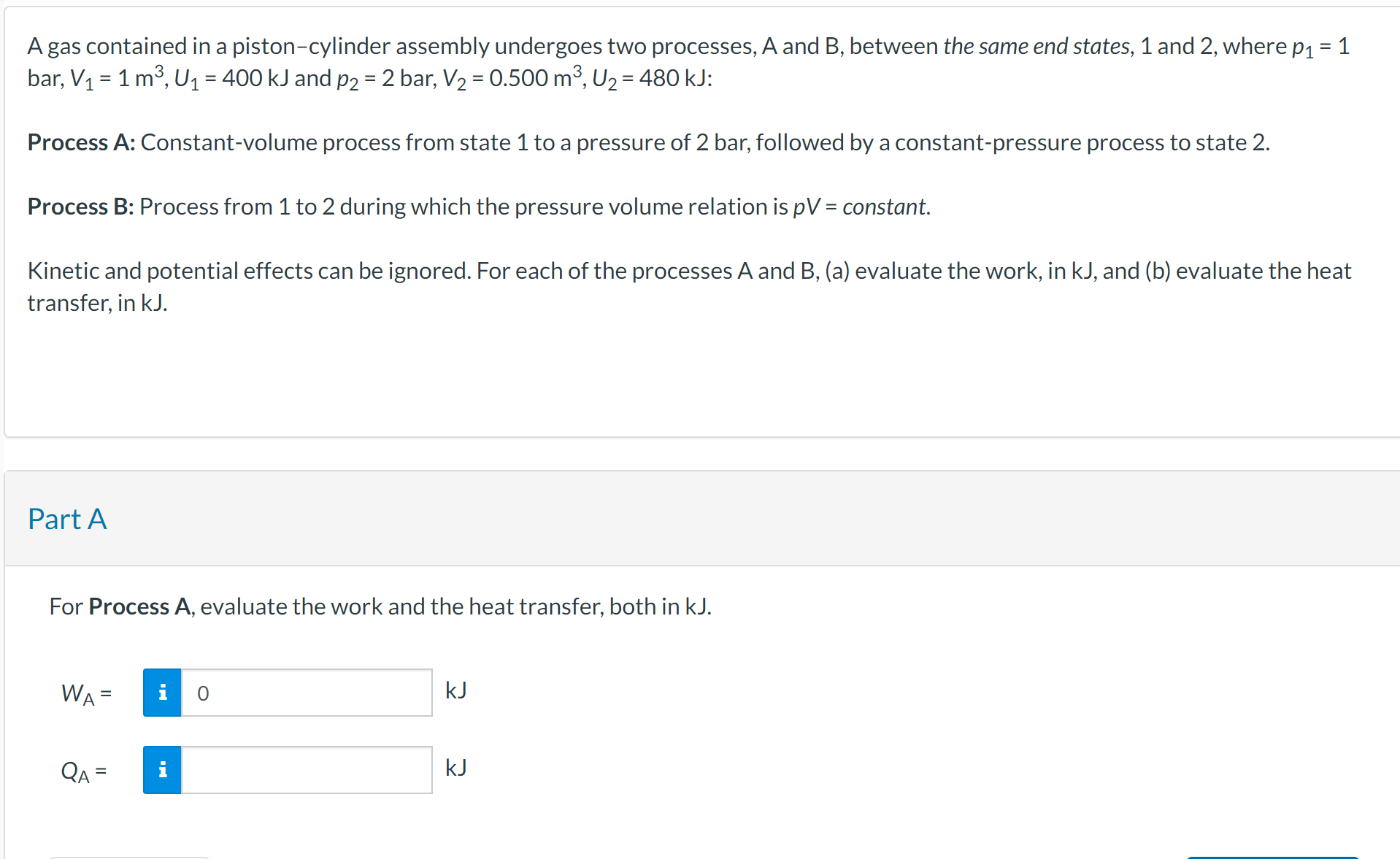
A gas contained in a piston-cylinder assembly undergoes two processes, A and B, between the same end states, 1 and 2, where p = 1 bar, V = 1 m, U = 400 kJ and p2 = 2 bar, V = 0.500 m, U = 480 kJ: Process A: Constant-volume process from state 1 to a pressure of 2 bar, followed by a constant-pressure process to state 2. Process B: Process from 1 to 2 during which the pressure volume relation is pV = constant. Kinetic and potential effects can be ignored. For each of the processes A and B, (a) evaluate the work, in kJ, and (b) evaluate the heat transfer, in kJ. Part A For Process A, evaluate the work and the heat transfer, both in kJ. WA QA = 0 KJ kJ
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Introduction to Chemical Engineering Thermodynamics
Authors: J. M. Smith, H. C. Van Ness, M. M. Abbott
7th edition
71247084, 978-0071247085
Students also viewed these Mechanical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



