Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A gas is contained in a cylinder fitted with a movable piston. The initial gas temperature is 25C. 1) The cylinder is placed in boiling
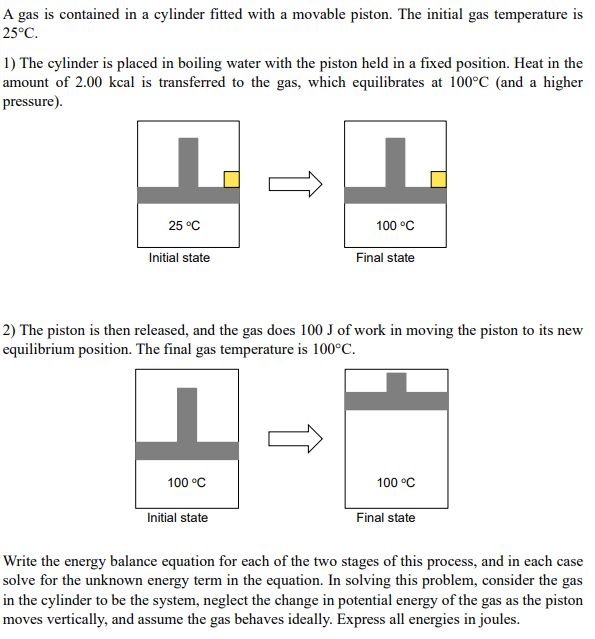
A gas is contained in a cylinder fitted with a movable piston. The initial gas temperature is 25C. 1) The cylinder is placed in boiling water with the piston held in a fixed position. Heat in the amount of 2.00kcal is transferred to the gas, which equilibrates at 100C (and a higher pressure). 2) The piston is then released, and the gas does 100J of work in moving the piston to its new equilibrium position. The final gas temperature is 100C. Write the energy balance equation for each of the two stages of this process, and in each case solve for the unknown energy term in the equation. In solving this problem, consider the gas in the cylinder to be the system, neglect the change in potential energy of the gas as the piston moves vertically, and assume the gas behaves ideally. Express all energies in joules
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


