Answered step by step
Verified Expert Solution
Question
1 Approved Answer
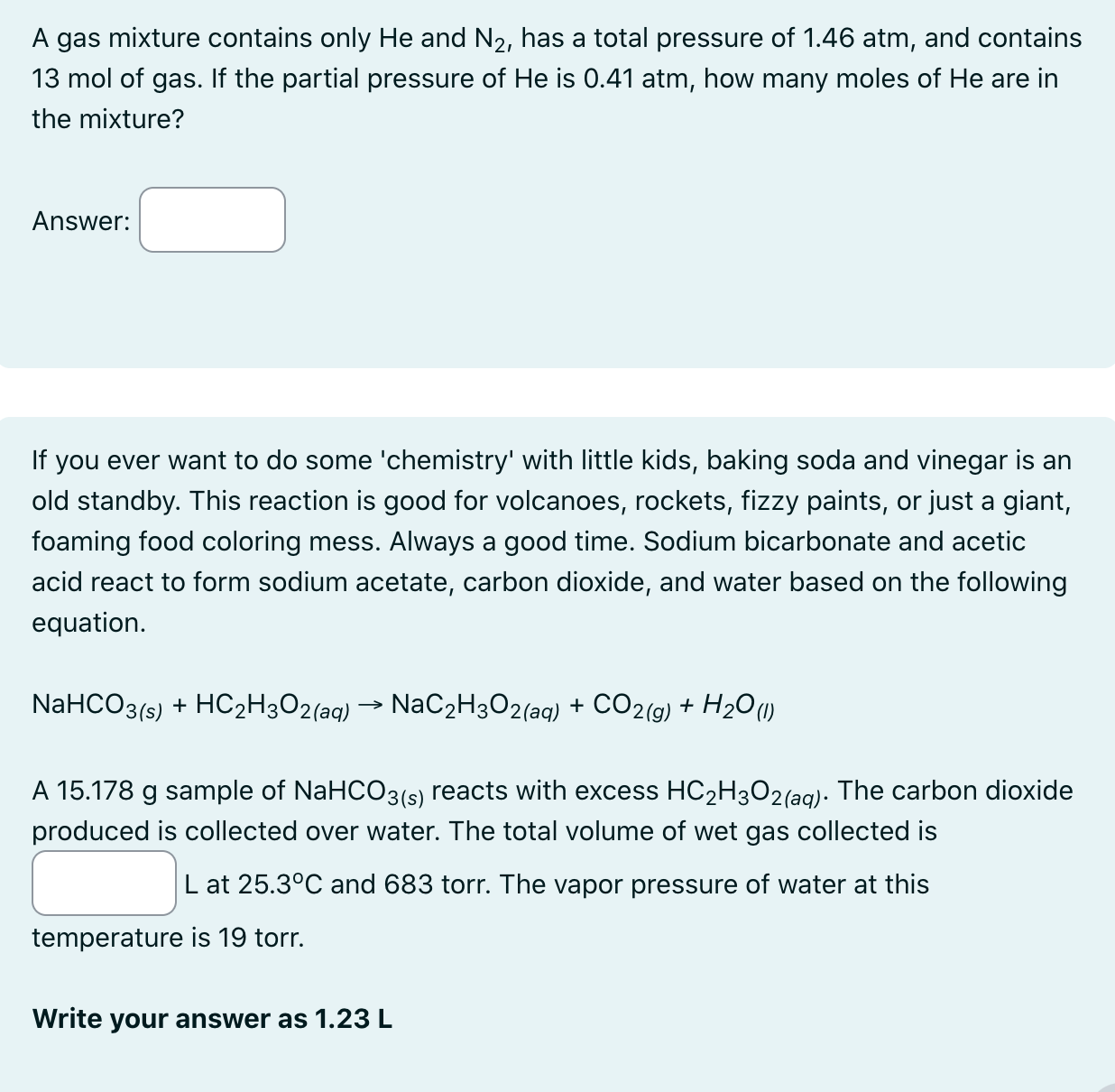
A gas mixture contains only H e and N 2 , has a total pressure of 1 . 4 6 atm, and contains 1 3
A gas mixture contains only and has a total pressure of atm, and contains
mol of gas. If the partial pressure of is atm, how many moles of are in
the mixture?
Answer:
If you ever want to do some 'chemistry' with little kids, baking soda and vinegar is an
old standby. This reaction is good for volcanoes, rockets, fizzy paints, or just a giant,
foaming food coloring mess. Always a good time. Sodium bicarbonate and acetic
acid react to form sodium acetate, carbon dioxide, and water based on the following
equation.
A sample of reacts with excess The carbon dioxide
produced is collected over water. The total volume of wet gas collected is
at and torr. The vapor pressure of water at this
temperature is torr.
Write your answer as
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


