Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A gas stream containing acetone in air flows from a solvent recovery unit at a rate of 142 L/s at 150.0 C and 1.3
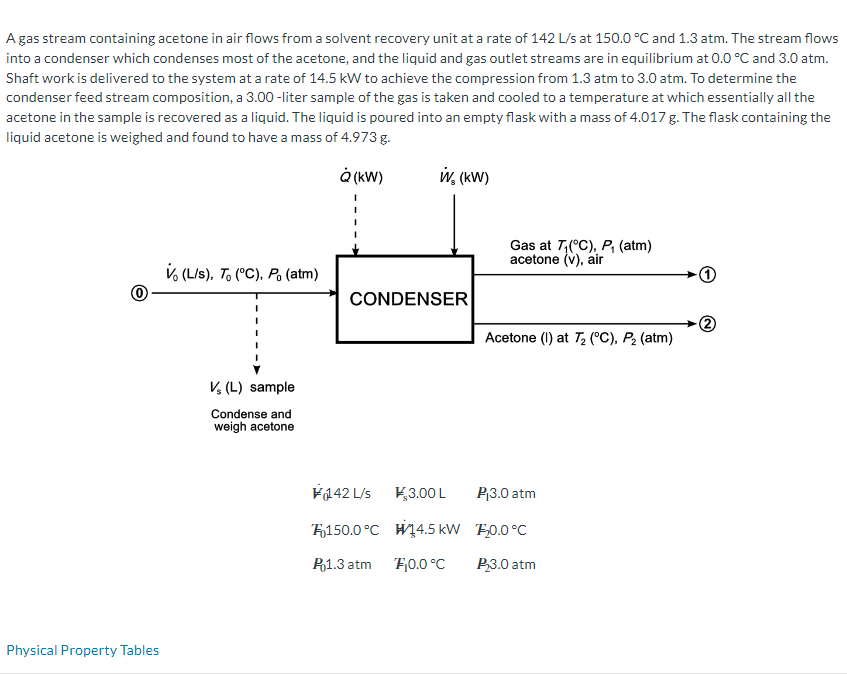
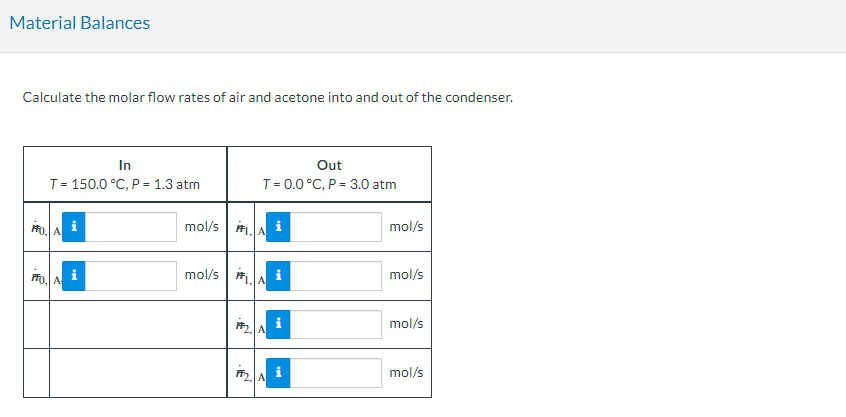
A gas stream containing acetone in air flows from a solvent recovery unit at a rate of 142 L/s at 150.0 C and 1.3 atm. The stream flows into a condenser which condenses most of the acetone, and the liquid and gas outlet streams are in equilibrium at 0.0 C and 3.0 atm. Shaft work is delivered to the system at a rate of 14.5 kW to achieve the compression from 1.3 atm to 3.0 atm. To determine the condenser feed stream composition, a 3.00-liter sample of the gas is taken and cooled to a temperature at which essentially all the acetone in the sample is recovered as a liquid. The liquid is poured into an empty flask with a mass of 4.017 g. The flask containing the liquid acetone is weighed and found to have a mass of 4.973 g. Physical Property Tables (kW) W, (kW) Gas at T(C), P (atm) acetone (V), air V (L/s), To (C). Po (atm) CONDENSER Acetone (1) at 72 (C), P (atm) Vs (L) sample Condense and weigh acetone 142 L/s *$3.00 L P3.0 atm 150.0C 14.5 kW 10.0C P1.3 atm F0.0C P3.0 atm Material Balances Calculate the molar flow rates of air and acetone into and out of the condenser. Out In T=150.0 C, P = 1.3 atm T= 0.0 C, P= 3.0 atm POA mol/s A mol/s mol/s A mol/s mol/s LA F < A mol/s
Step by Step Solution
There are 3 Steps involved in it
Step: 1
To solve this problem well perform the following steps 1 Calculate the Mass of Acetone in Sample Mass of empty flask 4017 g Mass of flask acetone 4973 ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


