Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A gaseous mixture consists of 10%N_(2),20%CO_(2),30%CO,20%H_(2) and 20%O_(2) (on a mass basis) at 260K and 10MPa . The mixture is contained in a piston-cylinder device
A gaseous mixture consists of
10%N_(2),20%CO_(2),30%CO,20%H_(2)and
20%O_(2)(on a mass basis) at
260Kand
10MPa. The mixture is contained in a piston-cylinder device and is heated at constant pressure until the temperature rises to
300K. Determine (a) the constant-pressure specific heat, (b) the apparent molar mass, (c) the apparent gas constant, (d) the heat transfer during the process by treating the mixture as an ideal gas (in
kJ) and (e) the heat transfer during the process by treating the mixture as a nonideal gas and using Amagat's law (in kJ).
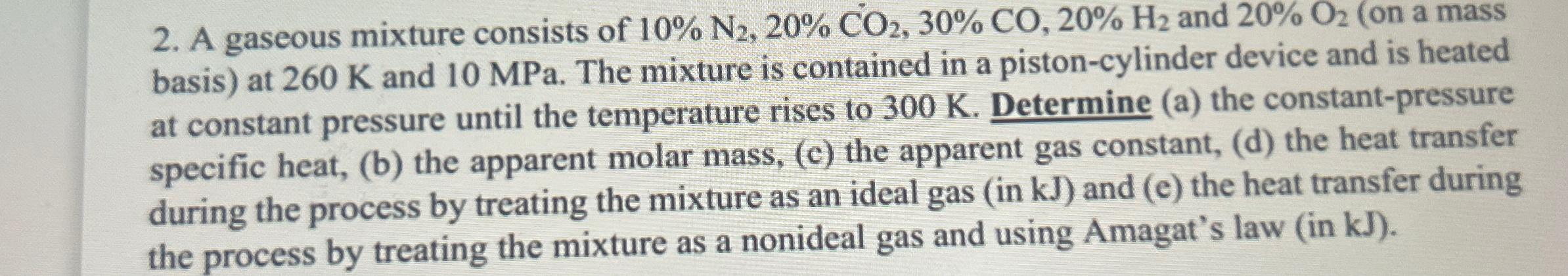
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


