Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A gaseous system undergoes a change in temperature and volume. What is the entropy change for a particle in this system if the final

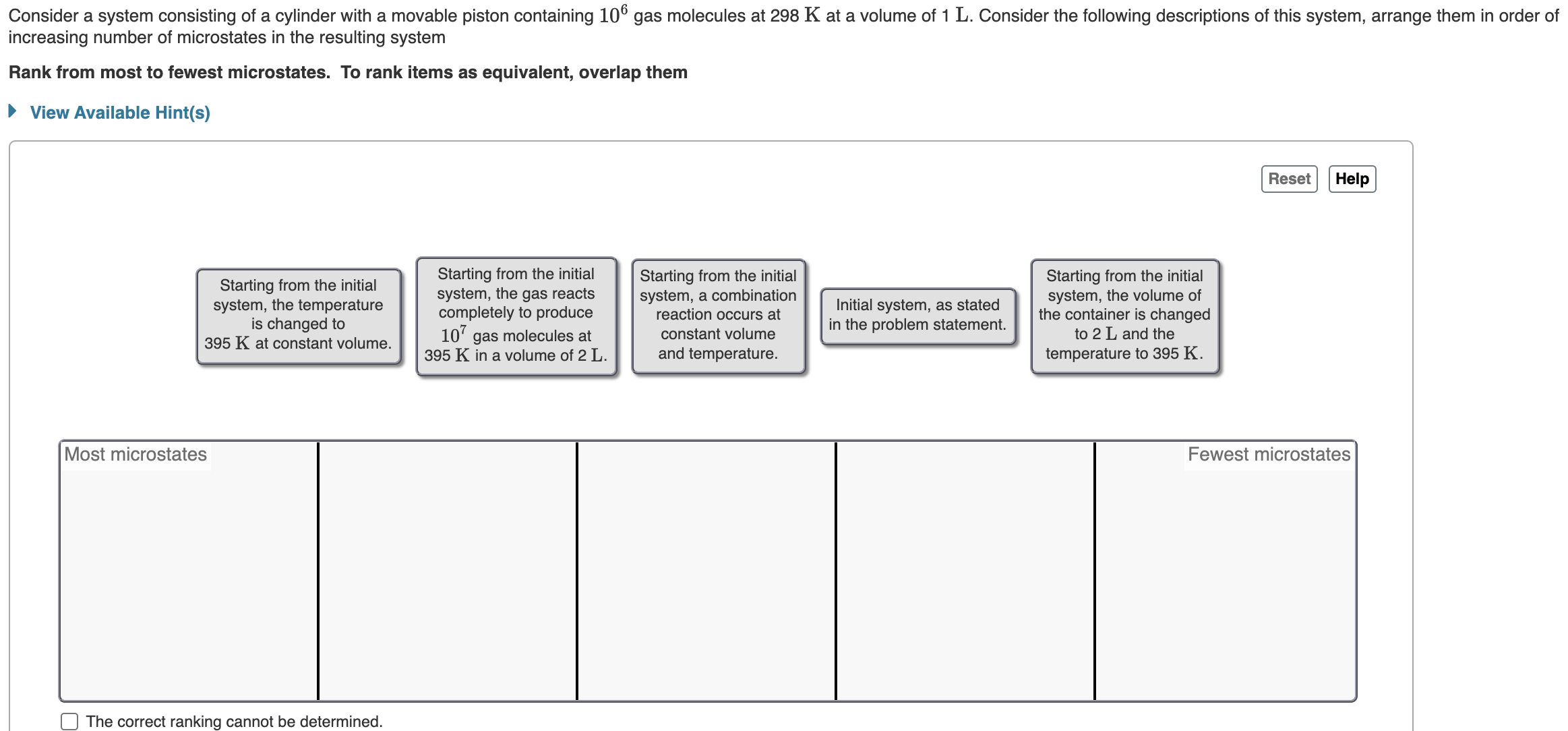
A gaseous system undergoes a change in temperature and volume. What is the entropy change for a particle in this system if the final number of microstates is 0.826 times that of the initial number of microstates? Express your answer numerically in joules per kelvin per particle. Consider a system consisting of a cylinder with a movable piston containing 106 gas molecules at 298 K at a volume of 1 L. Consider the following descriptions of this system, arrange them in order of increasing number of microstates in the resulting system Rank from most to fewest microstates. To rank items as equivalent, overlap them View Available Hint(s) Most microstates Starting from the initial system, the temperature is changed to 395 K at constant volume. The correct ranking cannot be determined. Starting from the initial system, the gas reacts completely to produce 107 gas molecules at 395 K in a volume of 2 L. Starting from the initial system, a combination reaction occurs at constant volume and temperature. Initial system, as stated in the problem statement. Starting from the initial system, the volume of the container is changed to 2 L and the temperature to 395 K. Reset Help Fewest microstates
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


