Question: A heat engine is based on a cycle consisting of an isobaric, an isometric and an adiabatic process (Fig. 2). The working substance is
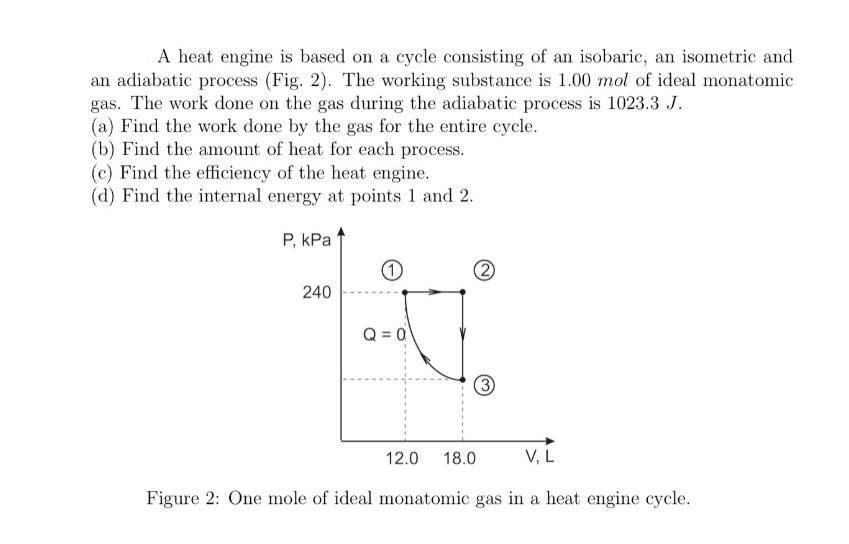
A heat engine is based on a cycle consisting of an isobaric, an isometric and an adiabatic process (Fig. 2). The working substance is 1.00 mol of ideal monatomic gas. The work done on the gas during the adiabatic process is 1023.3 J. (a) Find the work done by the gas for the entire cycle. (b) Find the amount of heat for each process. (c) Find the efficiency of the heat engine. (d) Find the internal energy at points 1 and 2. P, KPa 240 1 Q = 0 2 12.0 (3) V, L Figure 2: One mole of ideal monatomic gas in a heat engine cycle. 18.0
Step by Step Solution
There are 3 Steps involved in it
parta total work done is the area covered in the PV graph i here 1 ... View full answer

Get step-by-step solutions from verified subject matter experts


