Answered step by step
Verified Expert Solution
Question
1 Approved Answer
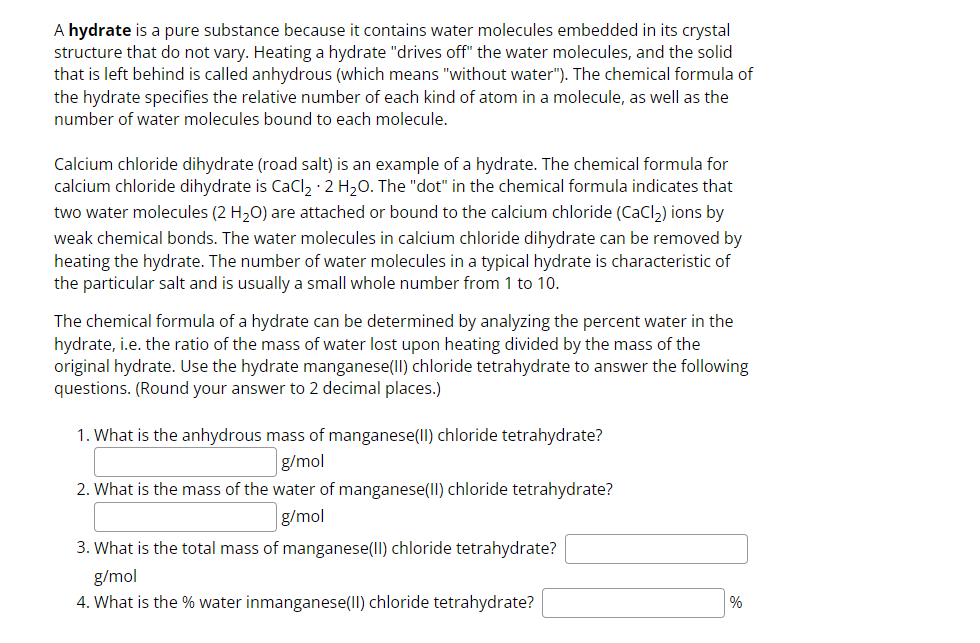
A hydrate is a pure substance because it contains water molecules embedded in its crystal structure that do not vary. Heating a hydrate drives off
A hydrate is a pure substance because it contains water molecules embedded in its crystal
structure that do not vary. Heating a hydrate "drives off" the water molecules, and the solid
that is left behind is called anhydrous which means "without water" The chemical formula of
the hydrate specifies the relative number of each kind of atom in a molecule, as well as the
number of water molecules bound to each molecule.
Calcium chloride dihydrate road salt is an example of a hydrate. The chemical formula for
calcium chloride dihydrate is The "dot" in the chemical formula indicates that
two water molecules are attached or bound to the calcium chloride ions by
weak chemical bonds. The water molecules in calcium chloride dihydrate can be removed by
heating the hydrate. The number of water molecules in a typical hydrate is characteristic of
the particular salt and is usually a small whole number from to
The chemical formula of a hydrate can be determined by analyzing the percent water in the
hydrate, ie the ratio of the mass of water lost upon heating divided by the mass of the
original hydrate. Use the hydrate manganeseII chloride tetrahydrate to answer the following
questions. Round your answer to decimal places.
What is the anhydrous mass of manganeseII chloride tetrahydrate?
What is the mass of the water of manganeseII chloride tetrahydrate?
What is the total mass of manganeseII chloride tetrahydrate?
What is the water inmanganeseII chloride tetrahydrate?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


