Answered step by step
Verified Expert Solution
Question
1 Approved Answer
(a) i. Draw a well labeled diagram of a fractionating column ii. Briefly explain the function of five component parts of a fractionating column (b)
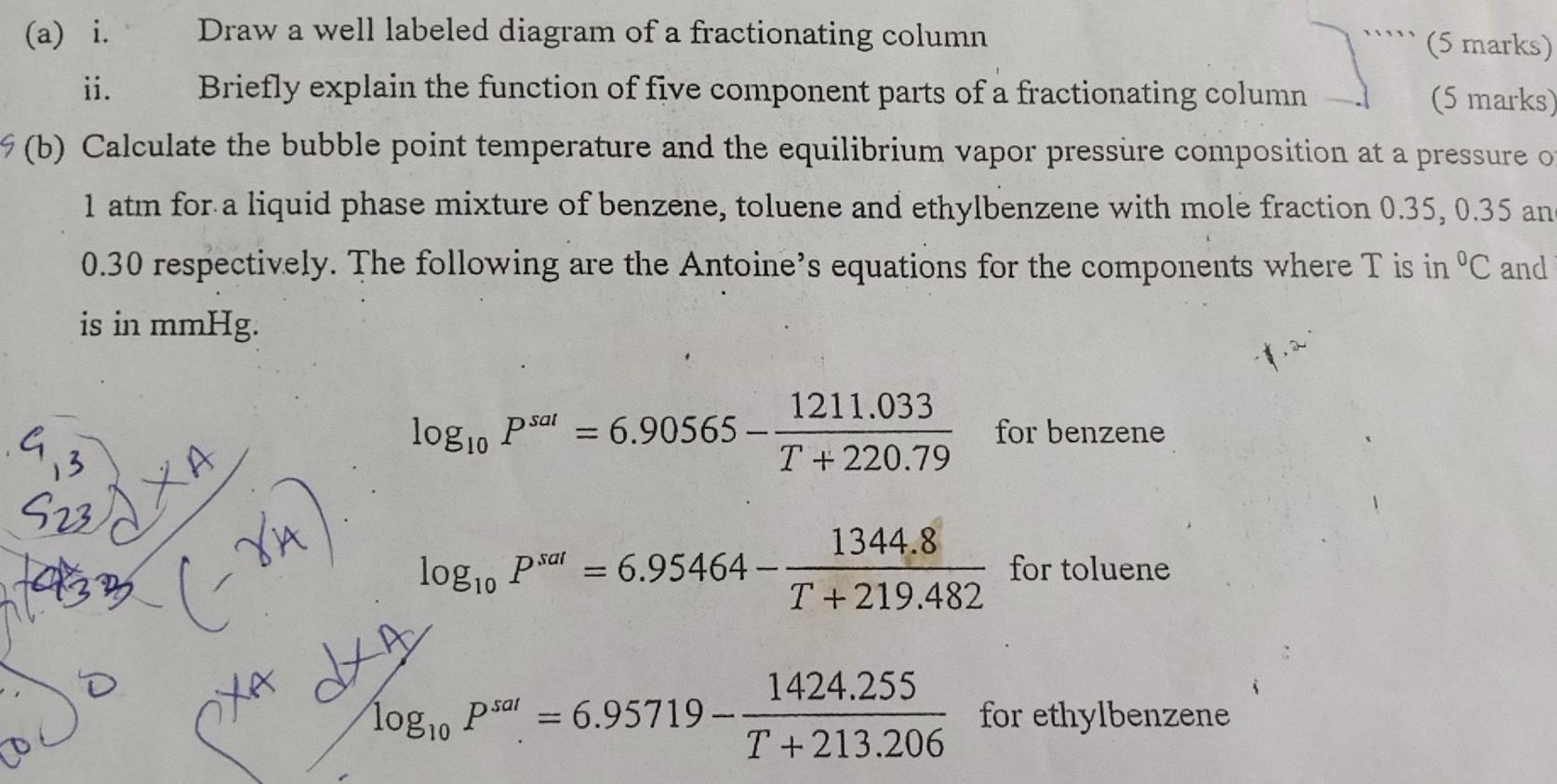
(a) i. Draw a well labeled diagram of a fractionating column ii. Briefly explain the function of five component parts of a fractionating column (b) Calculate the bubble point temperature and the equilibrium vapor pressure composition at a pressure 1 atm for a liquid phase mixture of benzene, toluene and ethylbenzene with mole fraction 0.35,0.35 an 0.30 respectively. The following are the Antoine's equations for the components where T is in C and is in mmHg. log10Psat=6.90565T+220.791211.033 for benzene log10Psat=6.95464T+219.4821344.8 for toluene log10Psat=6.95719T+213.2061424.255 for ethylbenzene
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


