Question
(a) (i) If in a kinetics experiment, the rate of hydrolysis of ethyl acetate (EtAc) was observed to proceed with a rate constant of
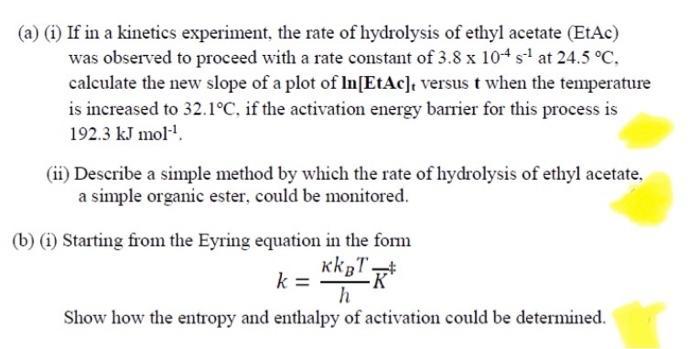
(a) (i) If in a kinetics experiment, the rate of hydrolysis of ethyl acetate (EtAc) was observed to proceed with a rate constant of 3.8 x 104s' at 24.5 C, calculate the new slope of a plot of In[EtAc], versus t when the temperature is increased to 32.1C, if the activation energy barrier for this process is 192.3 kJ mol". (ii) Describe a simple method by which the rate of hydrolysis of ethyl acetate, a simple organic ester, could be monitored. (b) (i) Starting from the Eyring equation in the form kk T. k = Show how the entropy and enthalpy of activation could be determined.
Step by Step Solution
3.41 Rating (164 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Organic Chemistry
Authors: Paula Yurkanis Bruice
4th edition
131407481, 978-0131407480
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



