Answered step by step
Verified Expert Solution
Question
1 Approved Answer
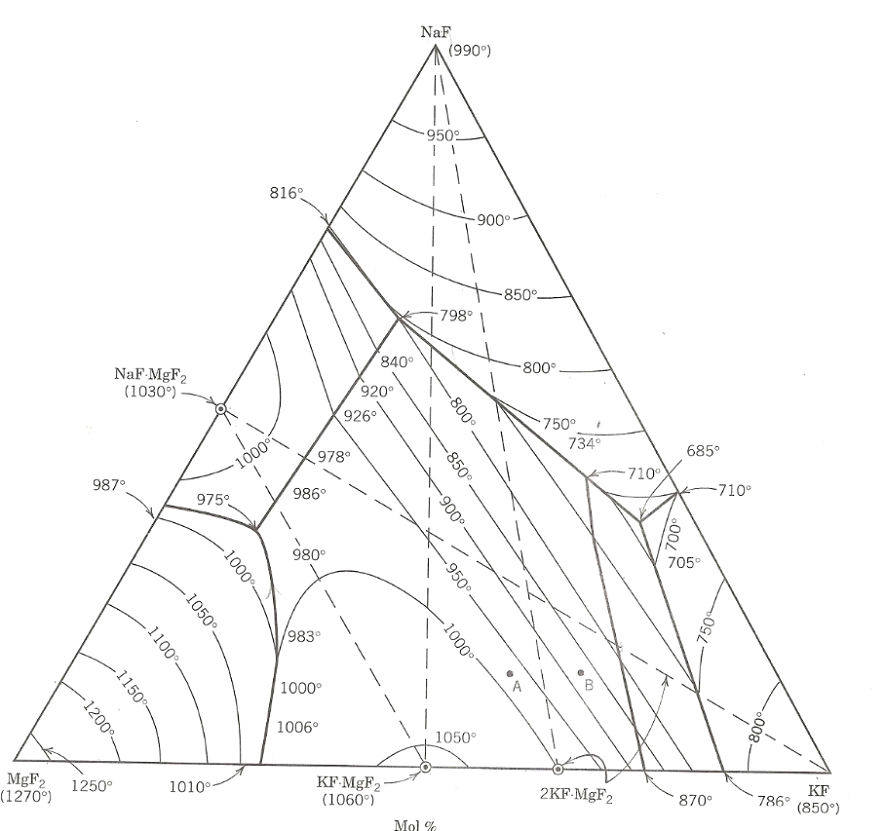
( a ) If a composition of 1 0 mole % KF , 1 0 mole % NaF, 8 0 mole % MgF , is
a If a composition of mole KF mole NaF, mole MgF is heated, at what temperature does it form a singlephase liquid?
b If a powder mixture of the three pure end members is prepared, what is the minimum temperature at which a liquid will form? Assume that a very sensitive calorimeter is used, which can detect the exotherm for forming very small amounts of liquid.
c Explain the crystallization path for a singlephase liquid of the composition marked A How does it differ from that marked B Is there an opportunity for nonequilibrium cooling in either case? Explain.
d For the composition A determine the phases present and the fractions of each phase at a temperatures of: deg Cdeg C and room temperature
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


