Question
A liquid fuel mixture consists of 20% ethanol and 80% n-octane (by volume) at 40C. Use the densities of ethanol and n-octane as 785
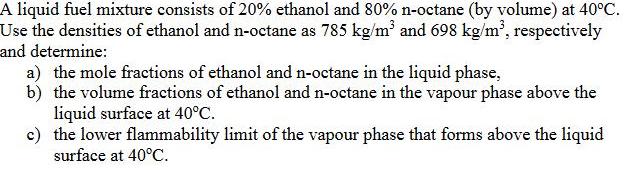
A liquid fuel mixture consists of 20% ethanol and 80% n-octane (by volume) at 40C. Use the densities of ethanol and n-octane as 785 kg/m' and 698 kg/m', respectively and determine: a) the mole fractions of ethanol and n-octane in the liquid phase, b) the volume fractions of ethanol and n-octane in the vapour phase above the liquid surface at 40C. c) the lower flammability limit of the vapour phase that forms above the liquid surface at 40C.
Step by Step Solution
3.55 Rating (159 Votes )
There are 3 Steps involved in it
Step: 1
Solution Volume percent of ethanol 20 Volume percent of noctanesor ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Elementary Principles of Chemical Processes
Authors: Richard M. Felder, Ronald W. Rousseau
3rd Edition
978-0471687573, 9788126515820, 978-0-471-4152, 0471720631, 047168757X, 8126515821, 978-0471720638
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



