Answered step by step
Verified Expert Solution
Question
1 Approved Answer
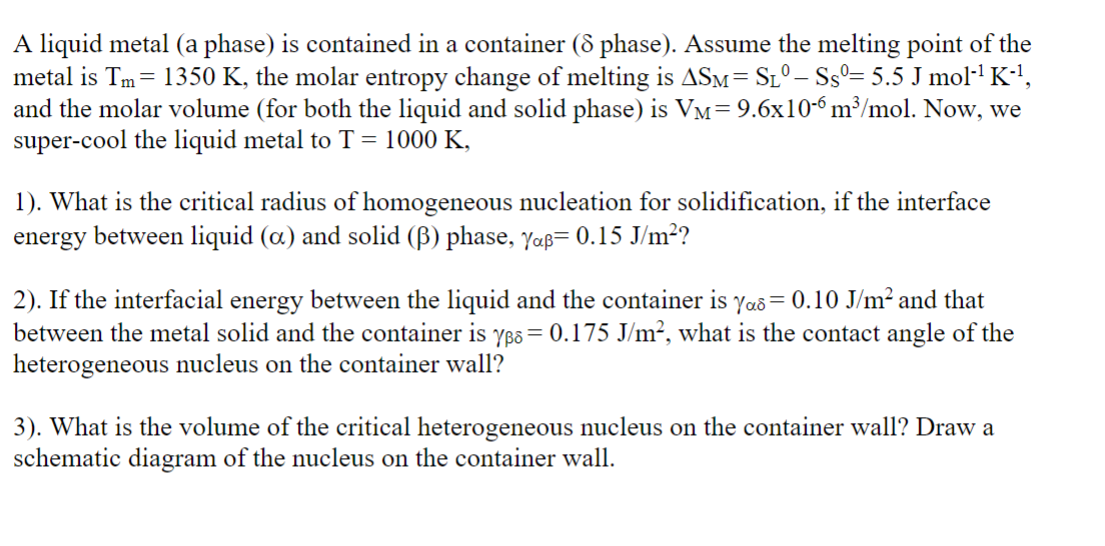
A liquid metal ( a phase ) is contained in a container ( phase ) . Assume the melting point of the metal is T
A liquid metal a phase is contained in a container phase Assume the melting point of the metal is the molar entropy change of melting is
and the molar volume for both the liquid and solid phase is Now, we
supercool the liquid metal to
What is the critical radius of homogeneous nucleation for solidification, if the interface energy between liquid and solid phase,
If the interfacial energy between the liquid and the container is and that between the metal solid and the container is what is the contact angle of the heterogeneous nucleus on the container wall?
What is the volume of the critical heterogeneous nucleus on the container wall? Draw a schematic diagram of the nucleus on the container wall.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


