Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A liquid mixture containing 30 mol% acetone (1) and 70% water (2) is flowing at 100 kmol/h. a) Calculate the bubble temperature of the mixture
A liquid mixture containing 30 mol% acetone (1) and 70% water (2) is flowing at 100 kmol/h.
a) Calculate the bubble temperature of the mixture when the flash drum is held at 1 bar.
b) Assume that the flash drum is at 1 bar. If the composition of the vapor product is to be y1 = 0.66, calculate the temperature of the flash chamber, the liquid phase composition, and V/F.
c) Does this system exhibit an azeotrope? If so, compute the temperature and composition at 1 bar.
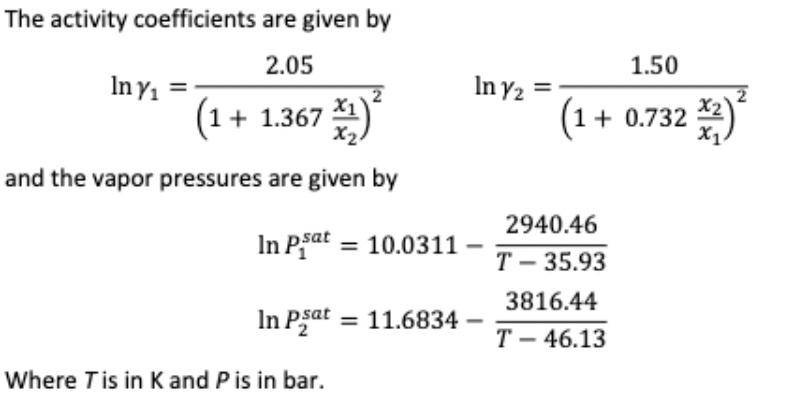
The activity coefficients are given by ln1=(1+1.367x2x1)22.05ln2=(1+0.732x1x2)21.50 and the vapor pressures are given by lnP1sat=10.0311T35.932940.46lnP2sat=11.6834T46.133816.44 Where T is in K and P is in barStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


