Answered step by step
Verified Expert Solution
Question
1 Approved Answer
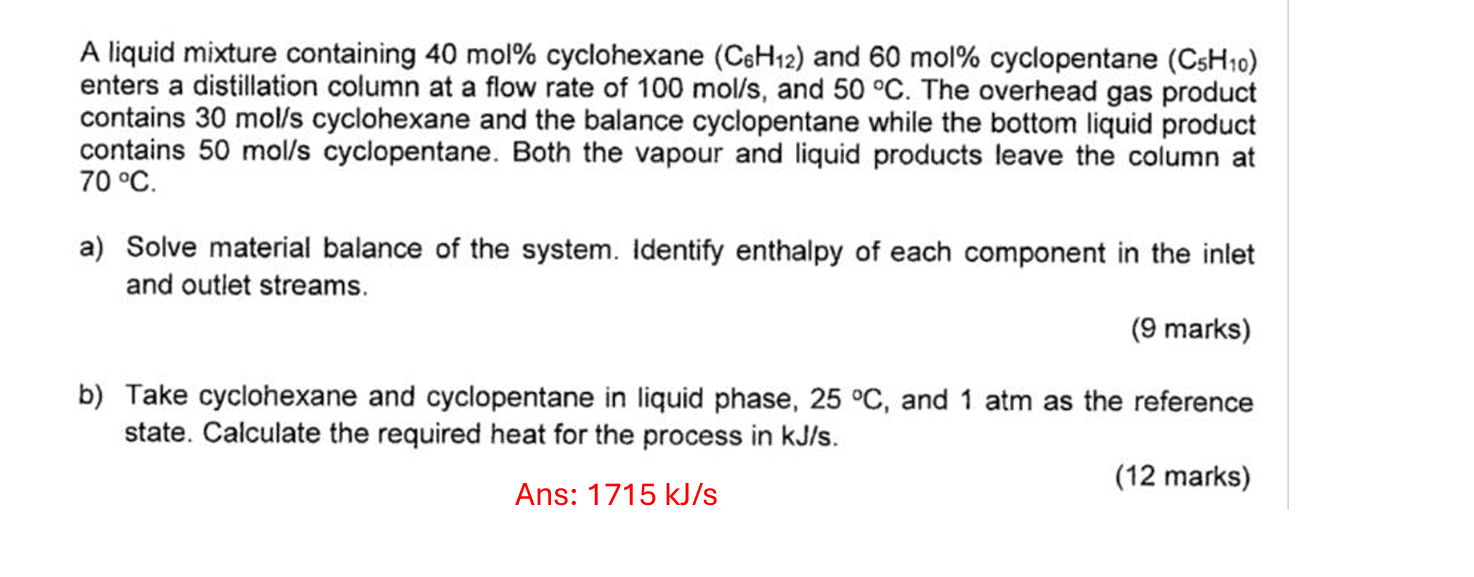
A liquid mixture containing 4 0 mol % cyclohexane ( C 6 H 1 2 ) and 6 0 mol % cyclopentane ( C 5
A liquid mixture containing mol cyclohexane and mol cyclopentane
enters a distillation column at a flow rate of and The overhead gas product
contains cyclohexane and the balance cyclopentane while the bottom liquid product
contains cyclopentane. Both the vapour and liquid products leave the column at
a Solve material balance of the system. Identify enthalpy of each component in the inlet
and outlet streams.
marks
b Take cyclohexane and cyclopentane in liquid phase, and atm as the reference
state. Calculate the required heat for the process in
Ans:
marks
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


