Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A liquid reaction A+B->C takes place in three CSTR's operating in series. The rate expression for this reaction is given to be as: -r_(A)=(k_(1)C_(A)C_(B))/(1+k_(2)C_(c)),(mo(l)/( l)t.min)
A liquid reaction
A+B->Ctakes place in three CSTR's operating in series. The rate\ expression for this reaction is given to be as:\
-r_(A)=(k_(1)C_(A)C_(B))/(1+k_(2)C_(c)),(mo(l)/( l)t.min) \ The concentration of
A,Band
Cat the inlet of the reactor are
C_(A0)=C_(B0)=1Mand\
C_(C0)=0. The volumetric flow rate of the inlet stream is
50l(t)/(m)in. All reactors operate at\
50\\\\deg C. The volume of the fist reactor is
50ltand the volume of the second and the third\ reactors are
75Iteach. Find the concentration of
Cat the outlet of the second and third\ reactors.\
k_(1)=10^(16)e^(-(12000)/(T))\ k_(2)=10^(7)e^(-(5000)/(T)) 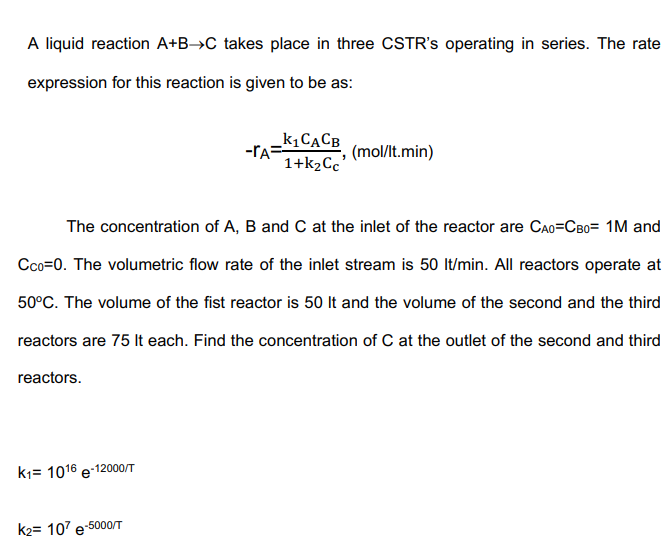
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


