Answered step by step
Verified Expert Solution
Question
1 Approved Answer
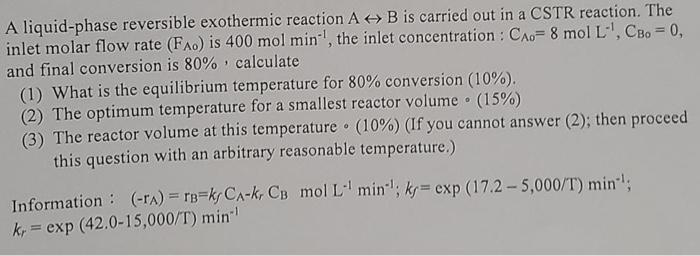
A liquid-phase reversible exothermic reaction A + B is carried out in a CSTR reaction. The inlet molar flow rate (FA0) is 400 mol min
A liquid-phase reversible exothermic reaction A + B is carried out in a CSTR reaction. The
inlet molar flow rate (FA0) is 400 mol min
, the inlet concentration: CA0= 8 mol L', CRO=0,
and final conversion is 80% calculate
(1) What is the equilibrium temperature for 80% conversion (10%).
(2) The optimum temperature for a smallest reactor volume (15%)
(3) The reactor volume at this temperature (10%) (If you cannot answer (2); then proceed
this question with an arbitrary reasonable temperature.)
Information: (-PA) = TD=kjCA-k-Ca mol L- min:; kJ= exp (17.2 - 5,000/T) min':
k, = exp (42.0-15,000/T) min-
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


