Question
(a) Lithium ion batteries with a LiCoO2 cathode and graphite anode are the most common batteries used in smart phone devices. (3+3+1+3+2=12) (i) Write
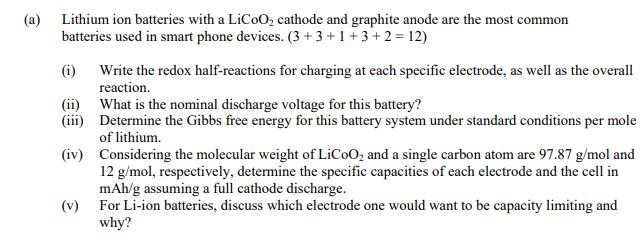
(a) Lithium ion batteries with a LiCoO2 cathode and graphite anode are the most common batteries used in smart phone devices. (3+3+1+3+2=12) (i) Write the redox half-reactions for charging at each specific electrode, as well as the overall reaction. (ii) What is the nominal discharge voltage for this battery? (iii) Determine the Gibbs free energy for this battery system under standard conditions per mole of lithium. (iv) Considering the molecular weight of LiCoO2 and a single carbon atom are 97.87 g/mol and 12 g/mol, respectively, determine the specific capacities of each electrode and the cell in mAh/g assuming a full cathode discharge. (v) For Li-ion batteries, discuss which electrode one would want to be capacity limiting and why?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Inorganic Chemistry
Authors: Mark Weller, Tina Overton, Jonathan Rourke
7th Edition
0198768125, 978-0198768128
Students also viewed these Marketing questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



